Deposition Date
2016-10-21
Release Date
2017-04-19
Last Version Date
2024-11-20
Entry Detail
PDB ID:
5TQ1
Keywords:
Title:
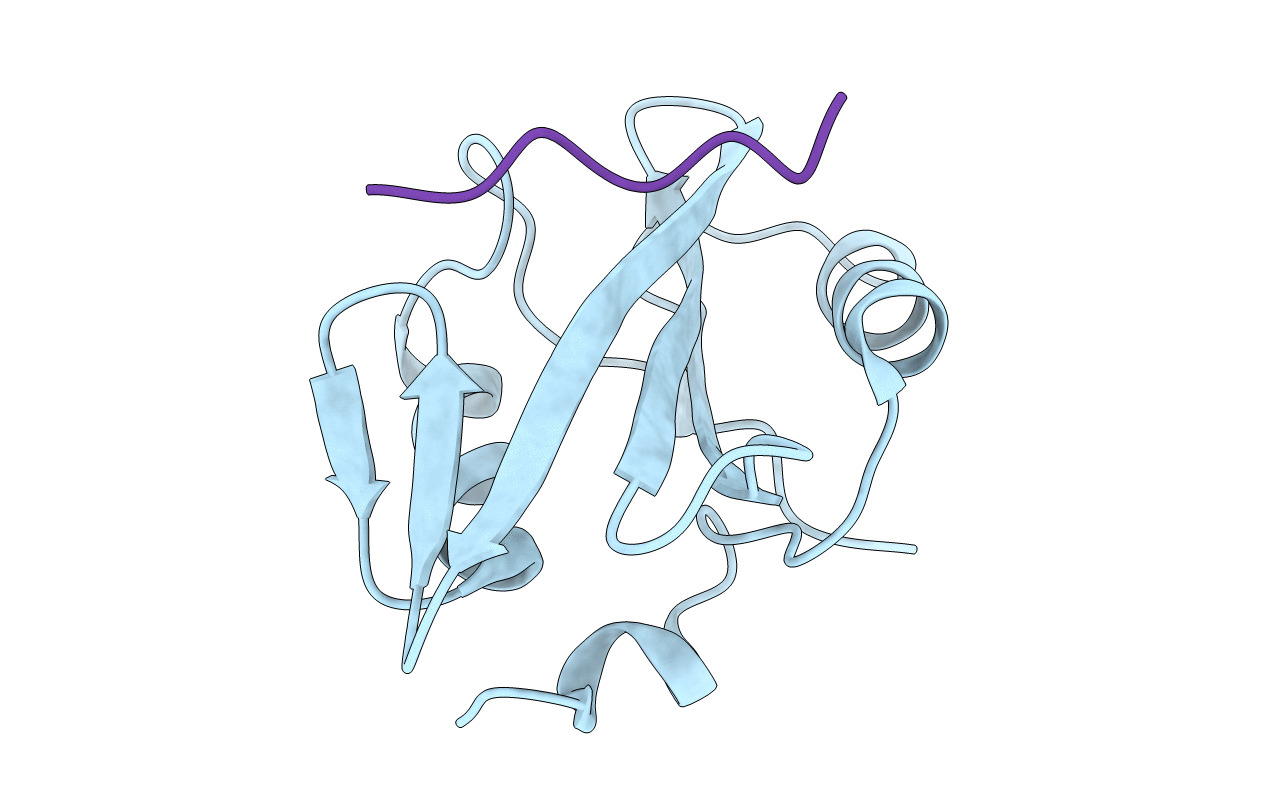
Phospholipase C gamma-1 C-terminal SH2 domain bound to a phosphopeptide derived from the insulin receptor
Biological Source:
Source Organism:
Bos taurus (Taxon ID: 9913)
Rattus norvegicus (Taxon ID: 10116)
Rattus norvegicus (Taxon ID: 10116)
Host Organism:
Method Details:
Experimental Method:
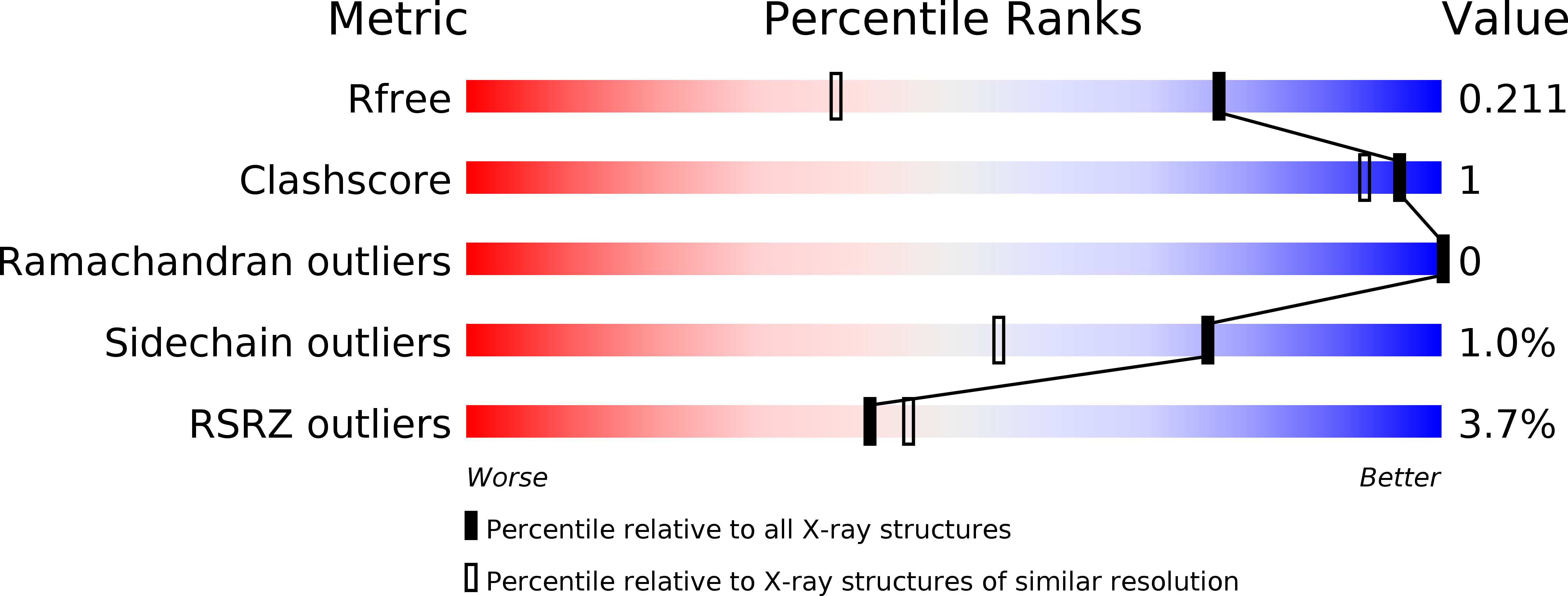
Resolution:
1.49 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 21