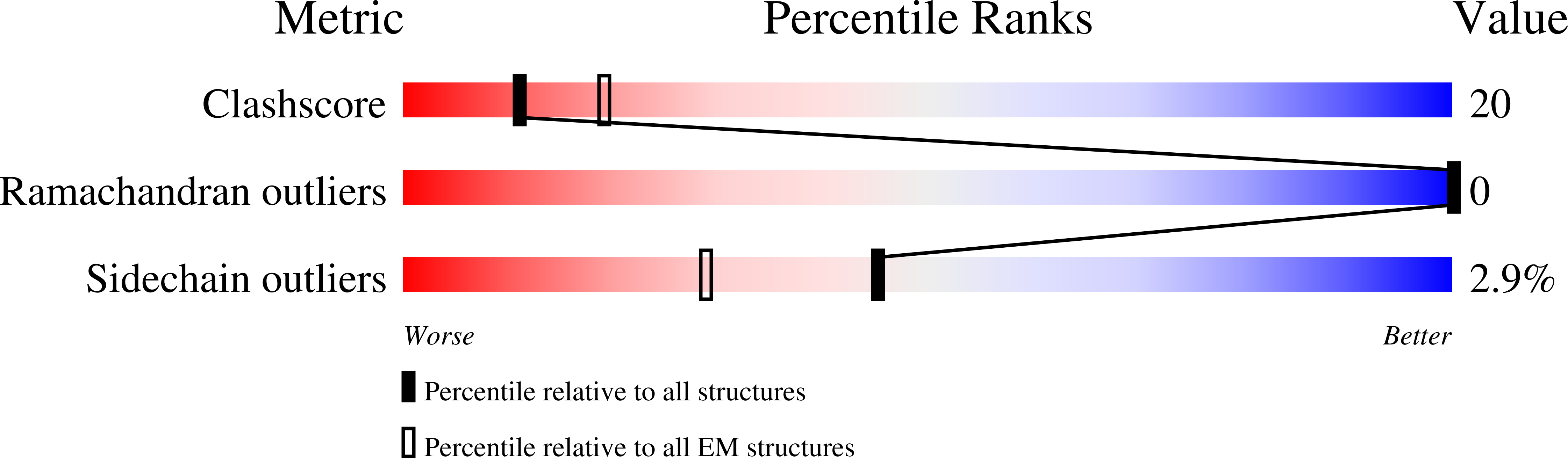
Deposition Date
2016-09-27
Release Date
2016-12-07
Last Version Date
2024-03-13
Entry Detail
PDB ID:
5TFY
Keywords:
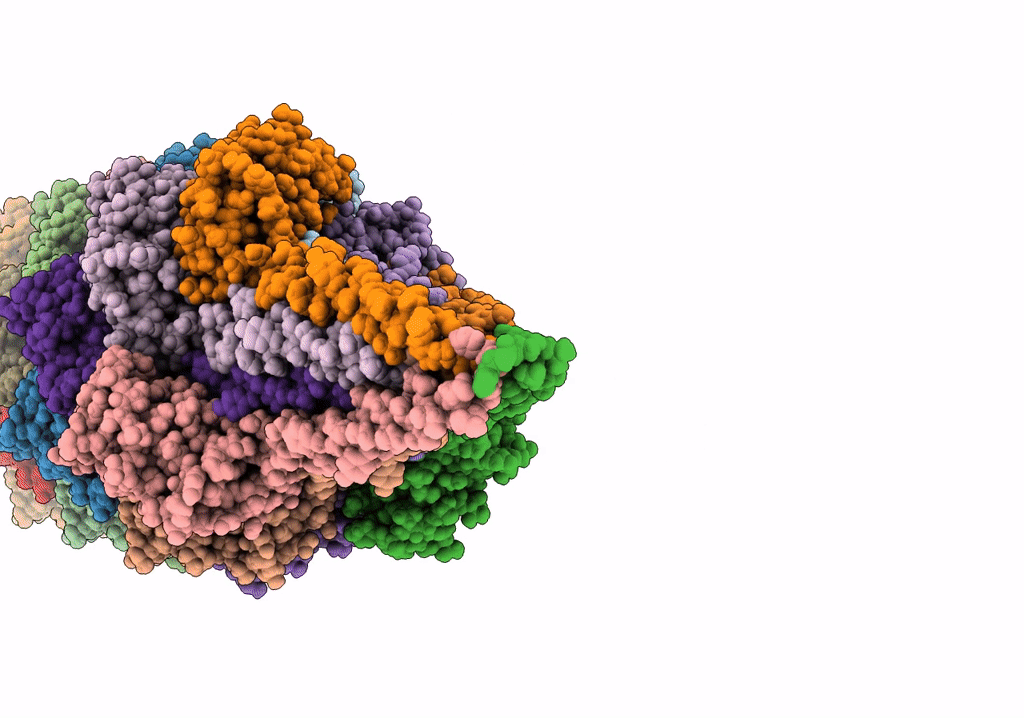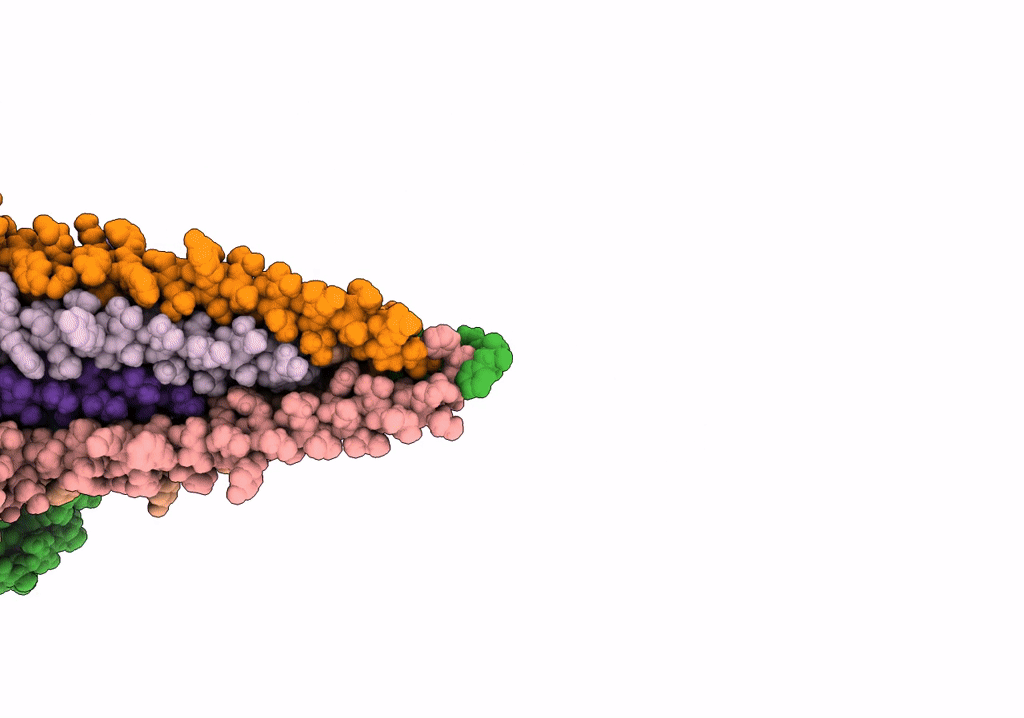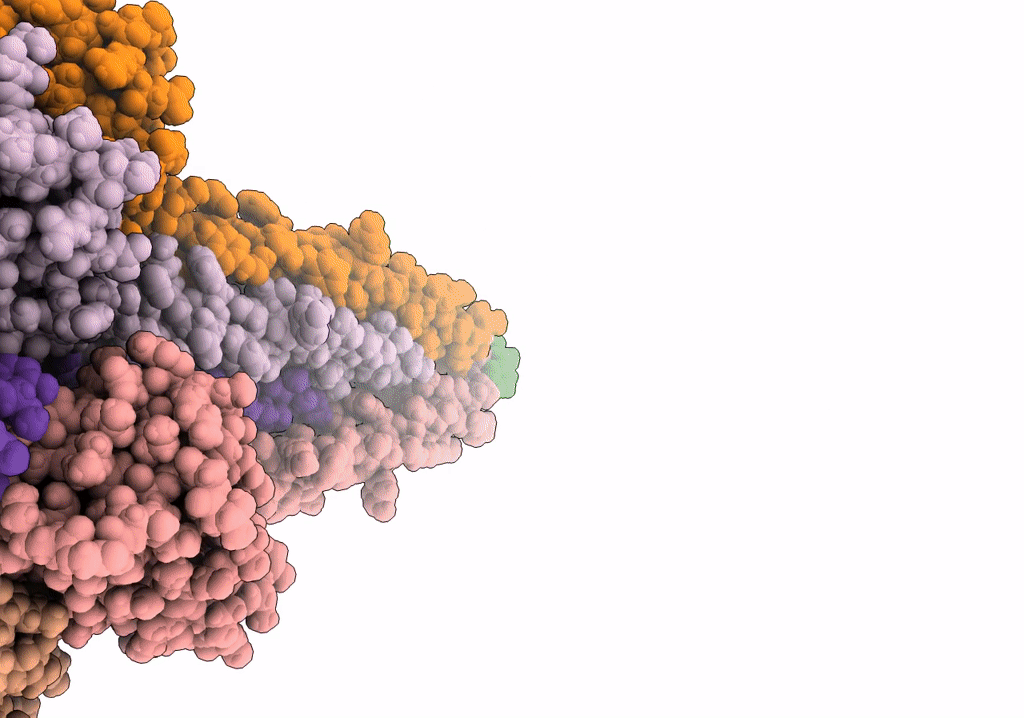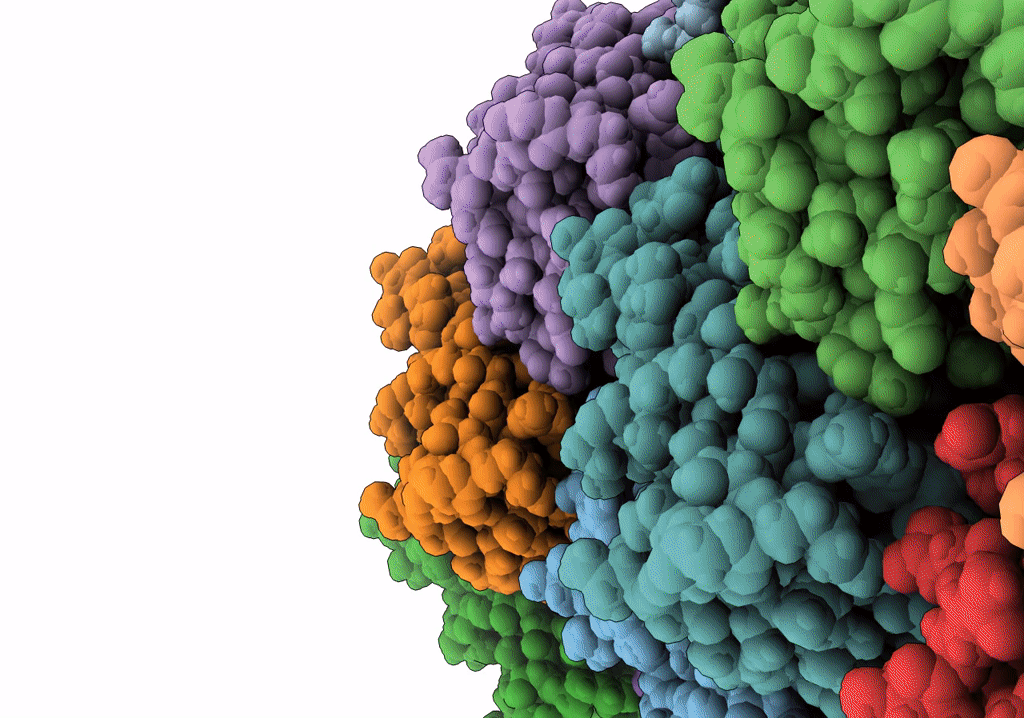
Title:
The archaeal flagellum of Methanospirillum hungatei strain JF1.
Biological Source:
Source Organism(s):
Method Details:
Experimental Method:
Resolution:
3.40 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL