Deposition Date
2016-09-11
Release Date
2017-01-11
Last Version Date
2023-10-25
Entry Detail
PDB ID:
5TB8
Keywords:
Title:
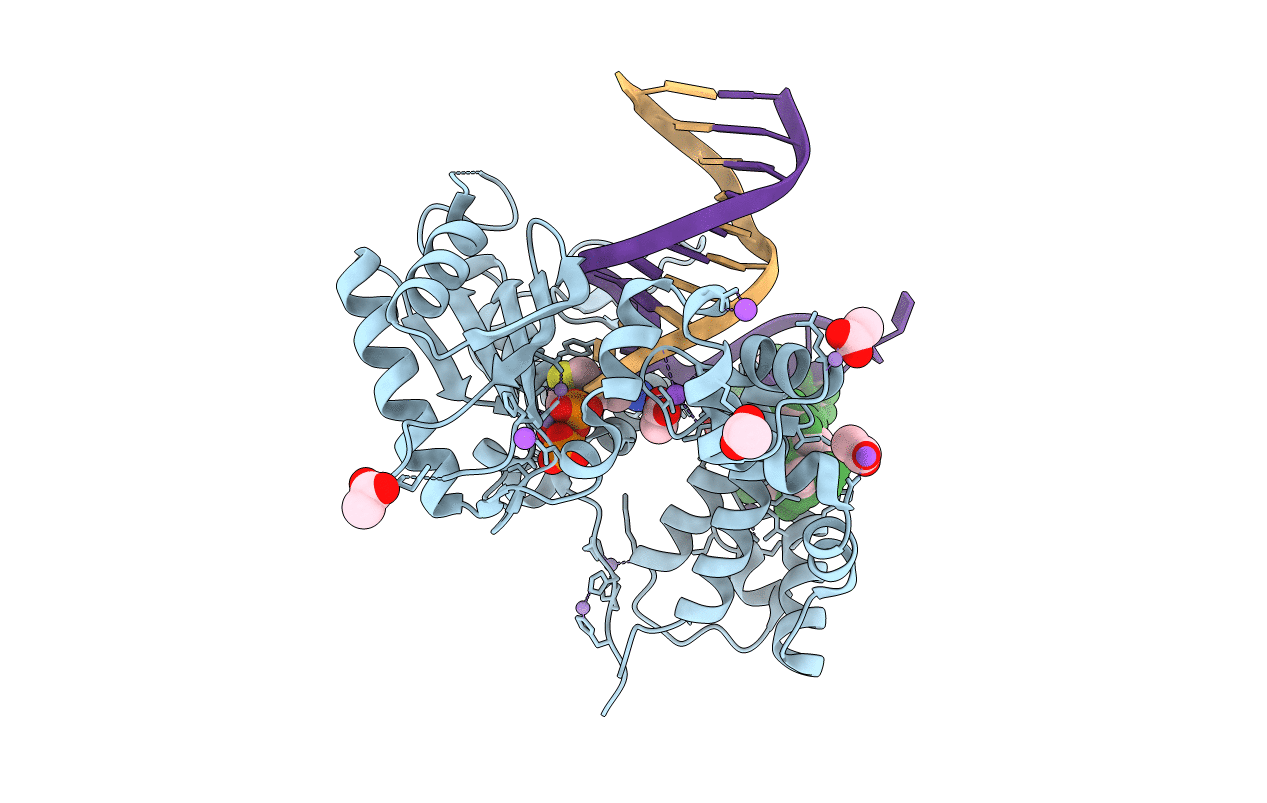
Precatalytic ternary complex of Human DNA Polymerase Beta in closed conformation With Gapped DNA substrate incoming (-)3TC-TP and Mn2+.
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Synthetic construct (Taxon ID: 32630)
Synthetic construct (Taxon ID: 32630)
Host Organism:
Method Details:
Experimental Method:
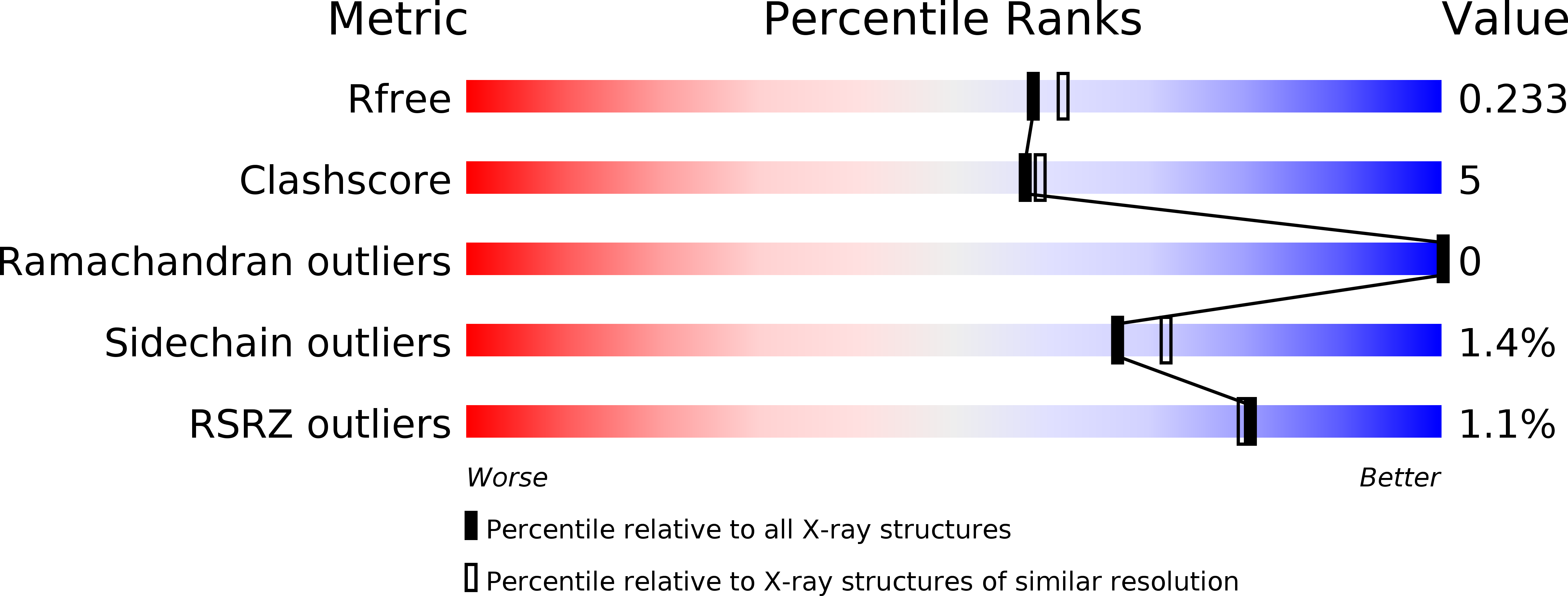
Resolution:
2.00 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 1 21 1