Deposition Date
2016-09-01
Release Date
2017-02-15
Last Version Date
2024-10-23
Entry Detail
PDB ID:
5T6Q
Keywords:
Title:
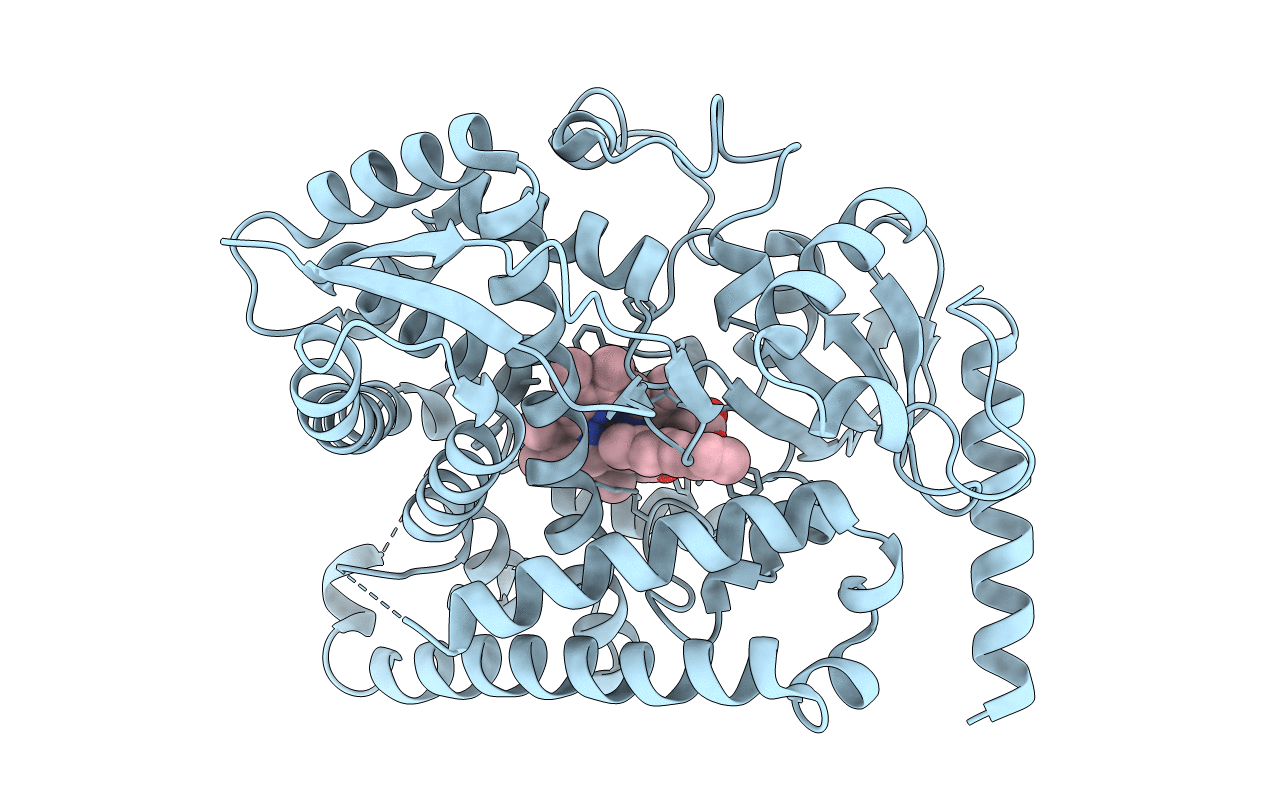
Structure of cytochrome P450 4B1 (CYP4B1) complexed with octane: An n-Alkane and fatty acid omega-hydroxylase with a covalently bound heme
Biological Source:
Source Organism:
Oryctolagus cuniculus (Taxon ID: 9986)
Host Organism:
Method Details:
Experimental Method:
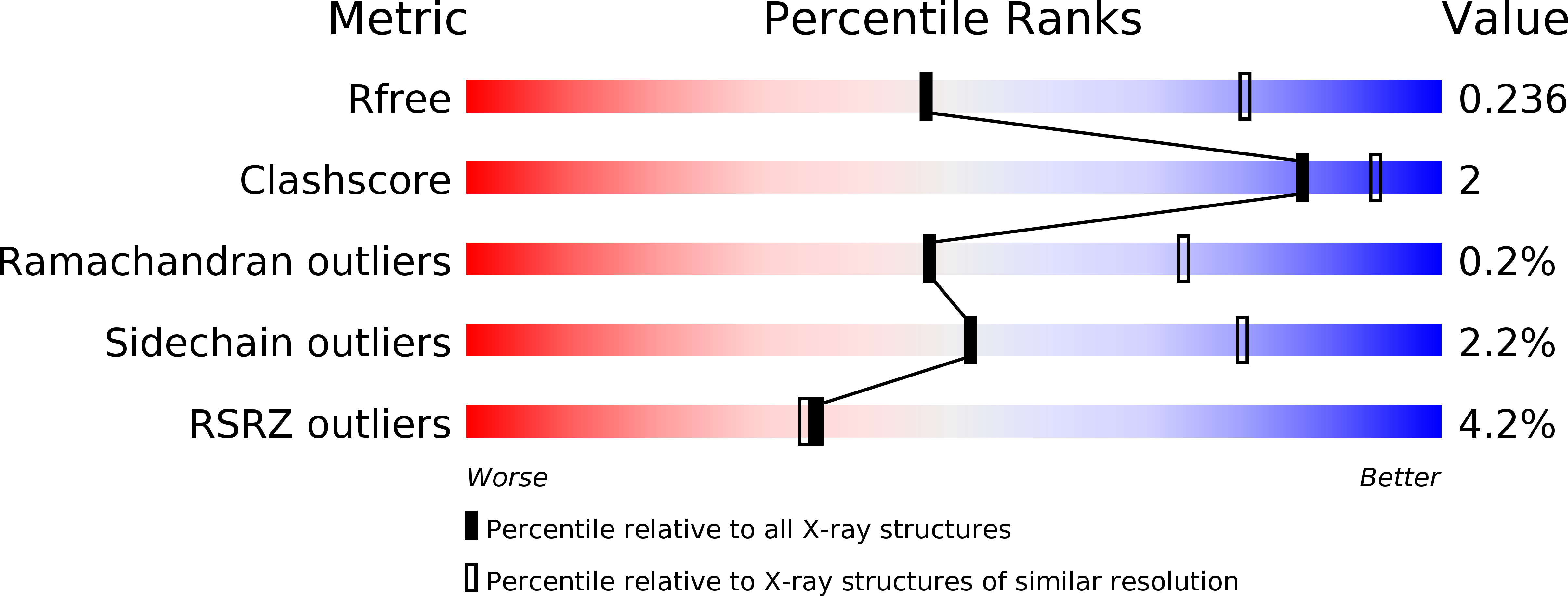
Resolution:
2.70 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 32 2 1