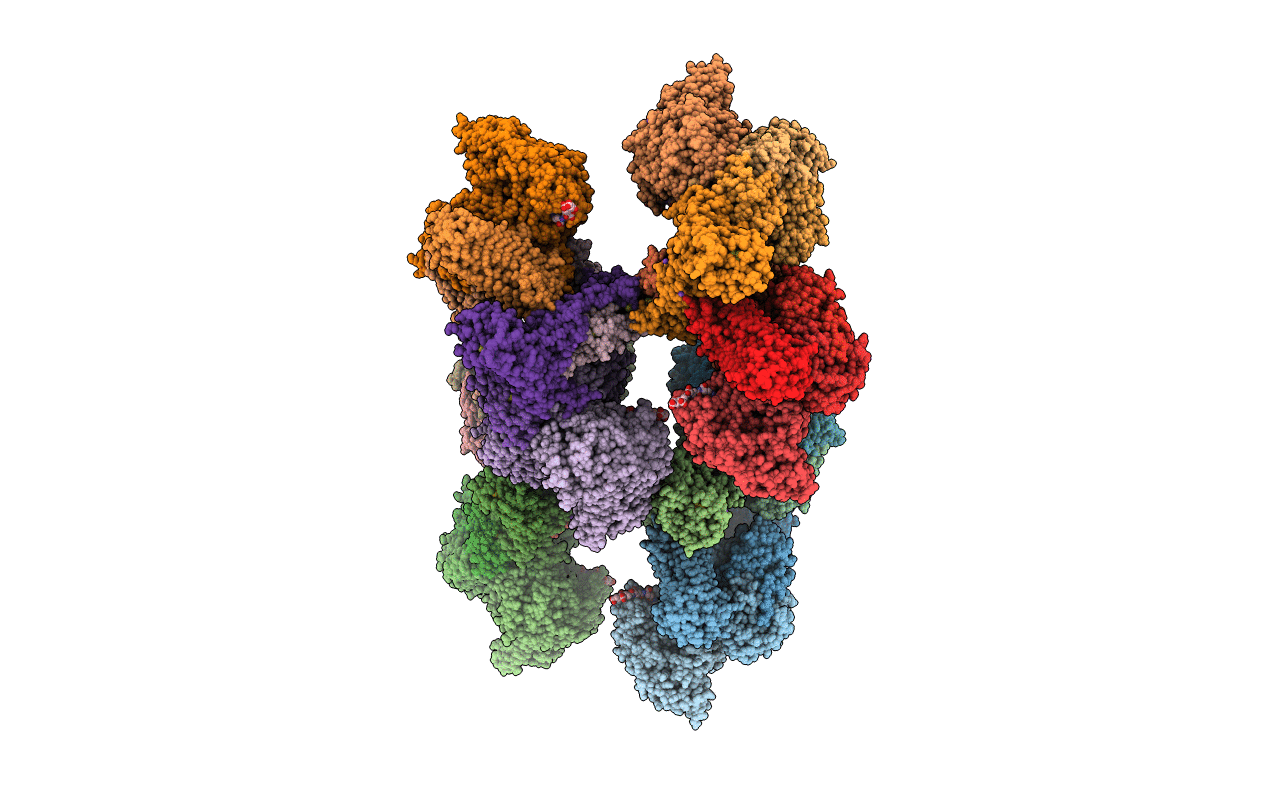
Deposition Date
2016-09-01
Release Date
2016-10-19
Last Version Date
2024-01-17
Entry Detail
PDB ID:
5T61
Keywords:
Title:
TUNGSTEN-CONTAINING FORMYLMETHANOFURAN DEHYDROGENASE FROM METHANOTHERMOBACTER WOLFEII, TRICLINIC FORM AT 2.55 A
Biological Source:
Source Organism(s):
Methanothermobacter wolfeii (Taxon ID: 145261)
Methanothermobacter sp. CaT2 (Taxon ID: 866790)
Methanothermobacter sp. CaT2 (Taxon ID: 866790)
Method Details:
Experimental Method:
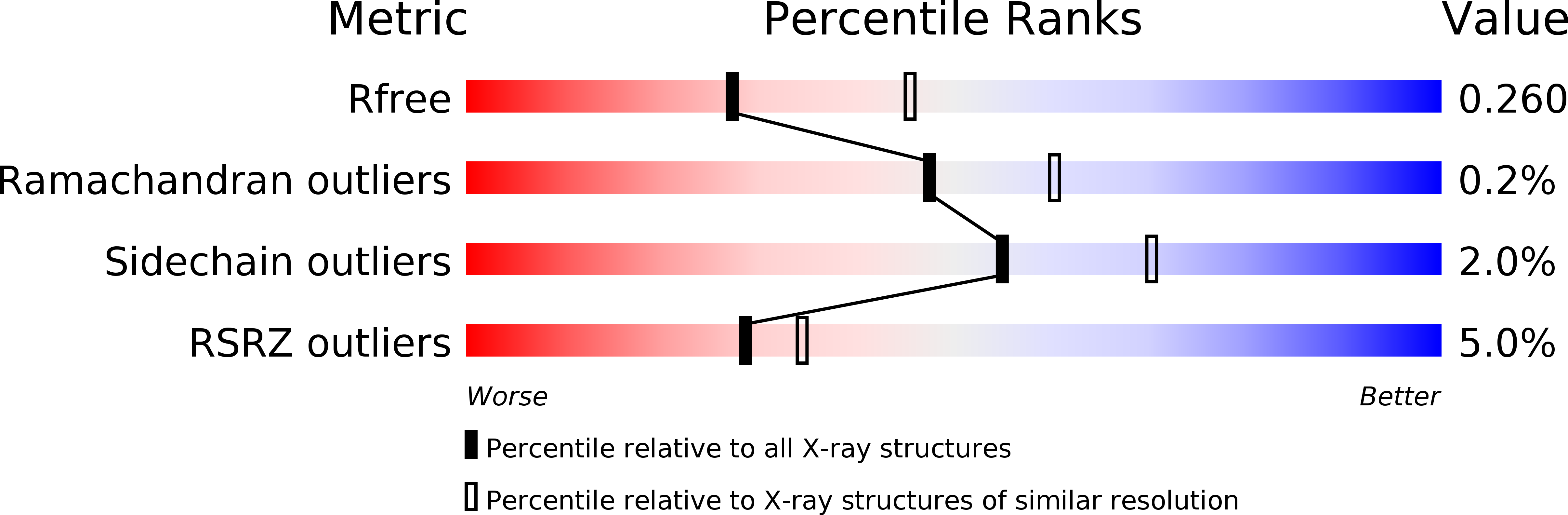
Resolution:
2.55 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.23
Space Group:
P 1