Deposition Date
1990-04-30
Release Date
1992-01-15
Last Version Date
2024-03-06
Entry Detail
PDB ID:
5P21
Keywords:
Title:
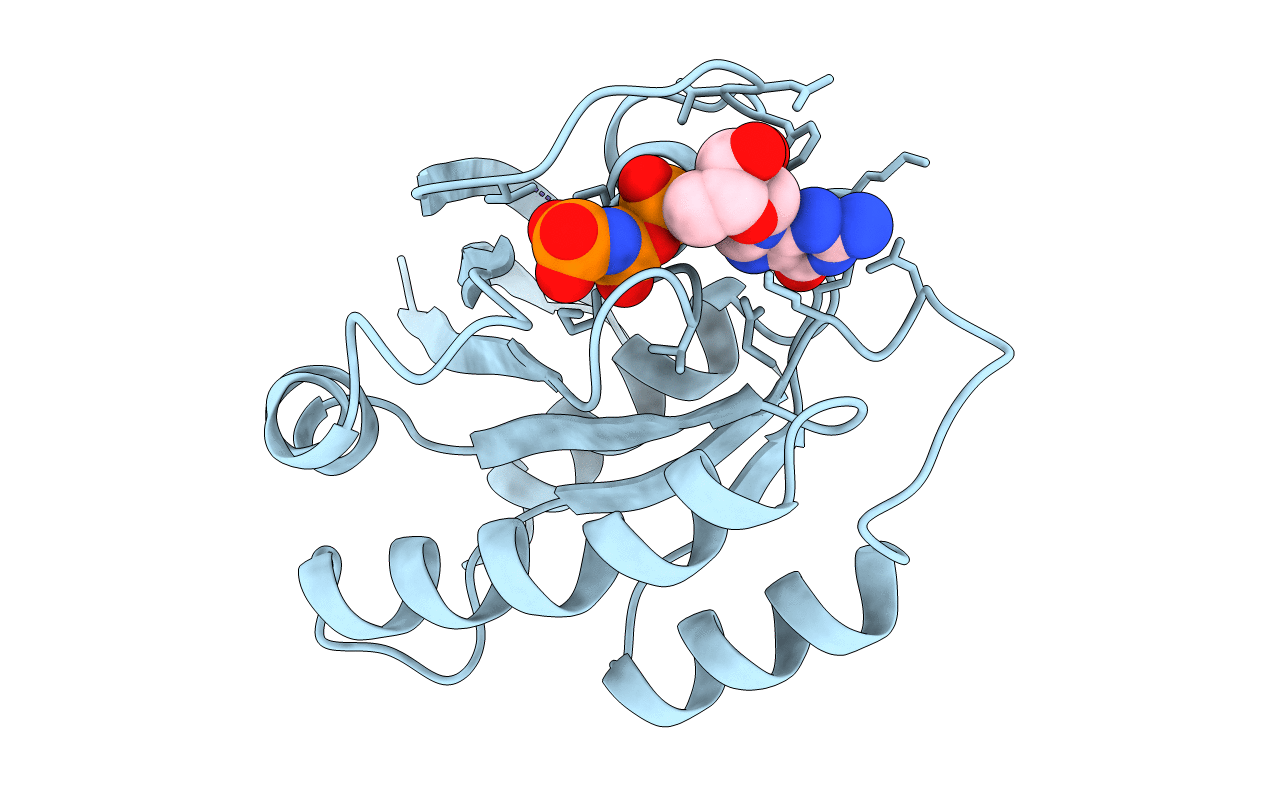
REFINED CRYSTAL STRUCTURE OF THE TRIPHOSPHATE CONFORMATION OF H-RAS P21 AT 1.35 ANGSTROMS RESOLUTION: IMPLICATIONS FOR THE MECHANISM OF GTP HYDROLYSIS
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details: