Deposition Date
2017-07-31
Release Date
2018-01-03
Last Version Date
2024-01-17
Entry Detail
PDB ID:
5OMK
Keywords:
Title:
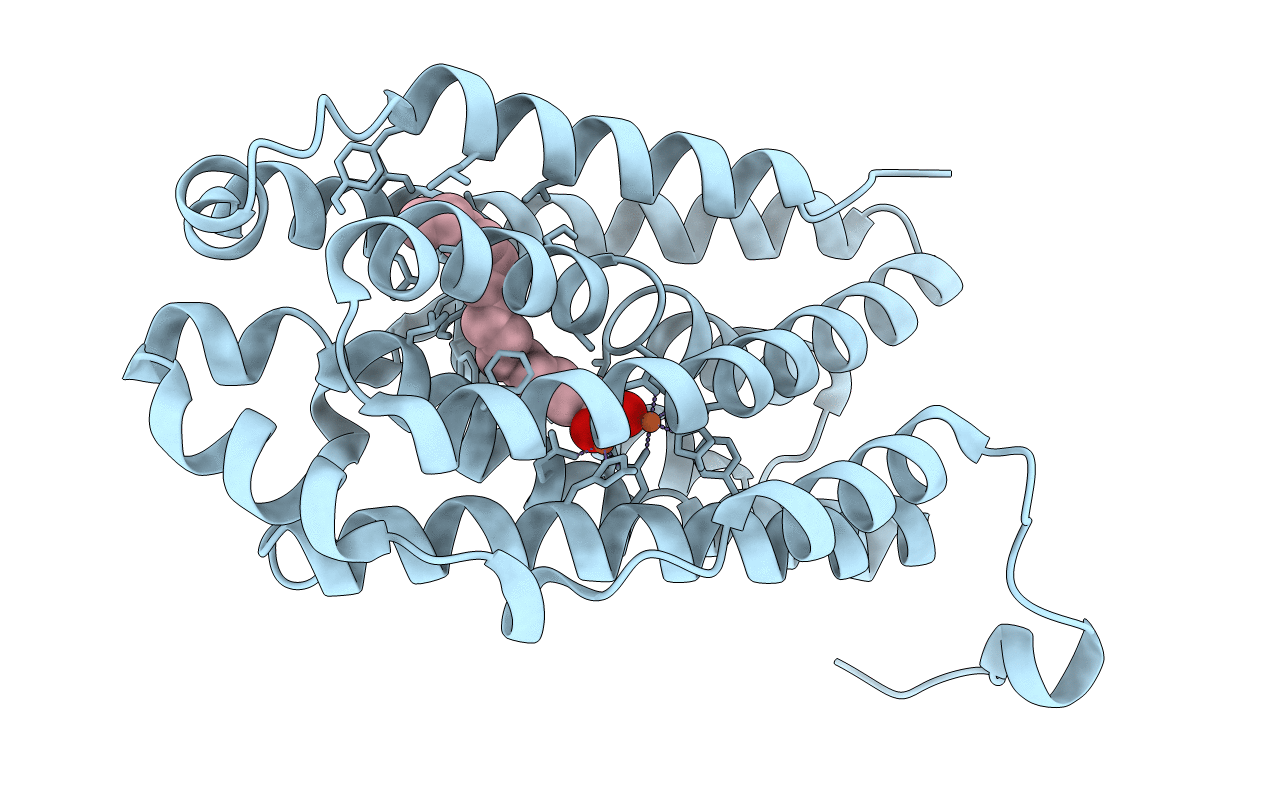
R2-like ligand-binding oxidase with aerobically reconstituted metal cofactor before photoconversion
Biological Source:
Source Organism(s):
Geobacillus kaustophilus (strain HTA426) (Taxon ID: 235909)
Expression System(s):
Method Details:
Experimental Method:
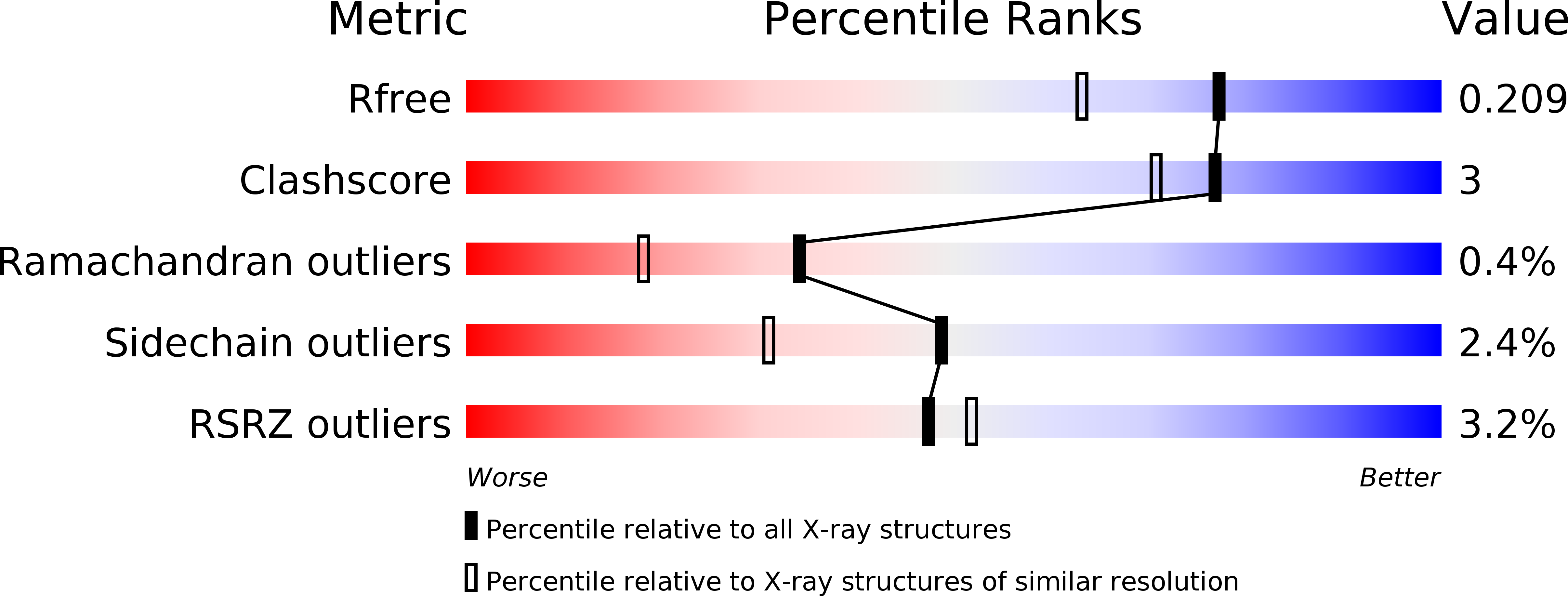
Resolution:
1.70 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
I 2 2 2