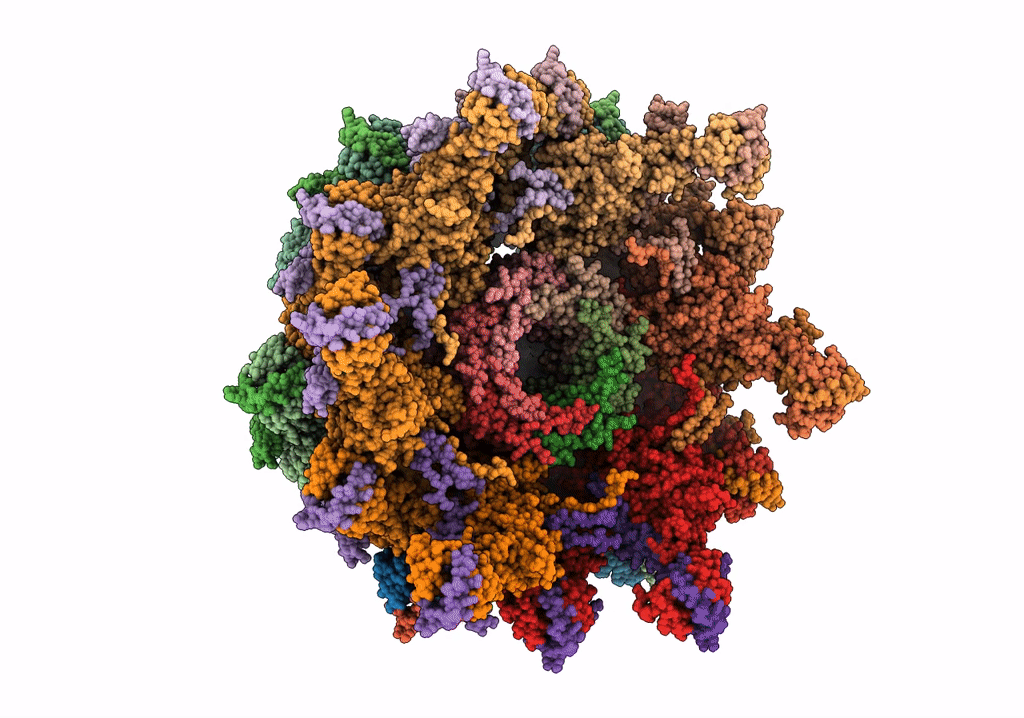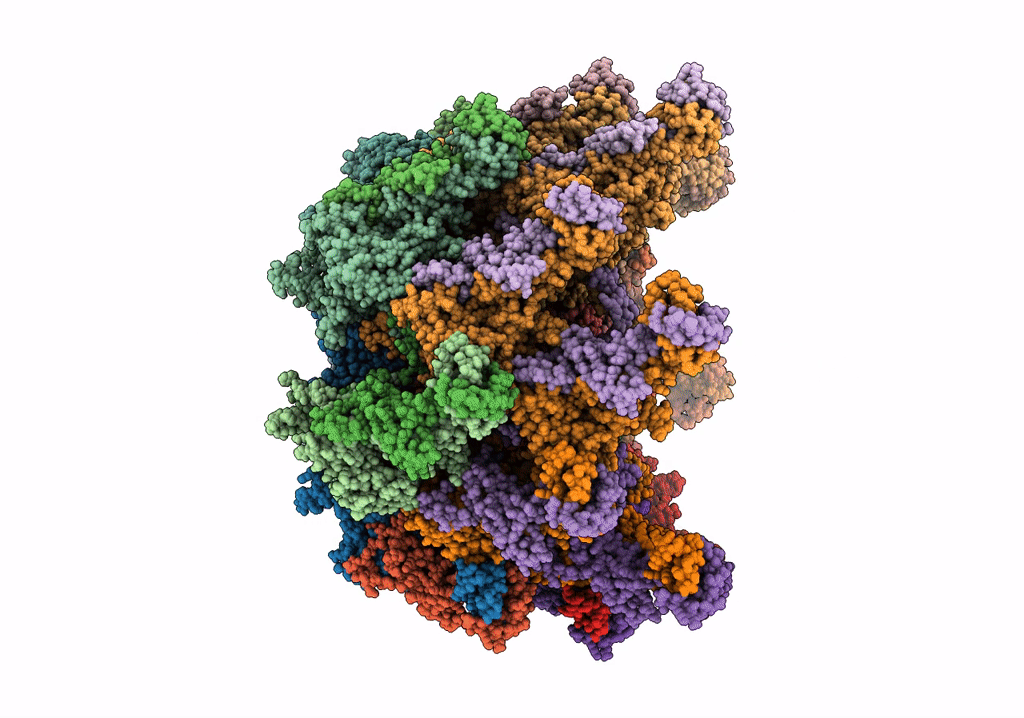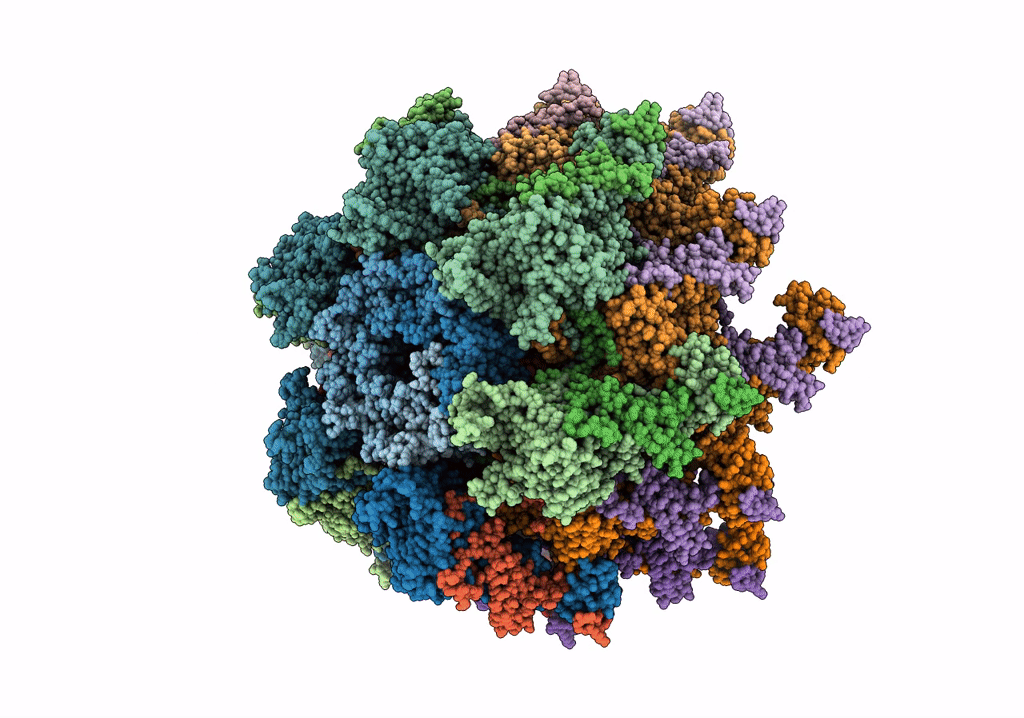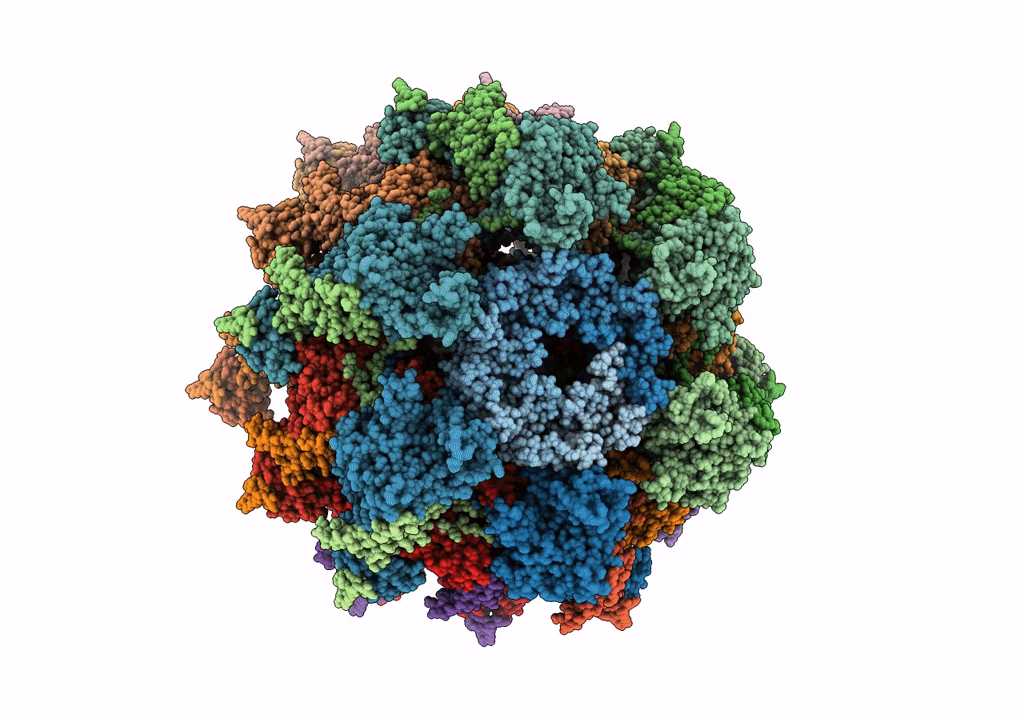
Deposition Date
2017-07-22
Release Date
2017-08-09
Last Version Date
2024-05-08
Entry Detail
PDB ID:
5OJQ
Keywords:
Title:
The modeled structure of of wild type extended type VI secretion system sheath/tube complex in vibrio cholerae based on cryo-EM reconstruction of the non-contractile sheath/tube complex
Biological Source:
Source Organism:
Vibrio cholerae (Taxon ID: 666)
Method Details:
Experimental Method:
Resolution:
3.70 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
HELICAL


