Deposition Date
2017-07-13
Release Date
2017-11-22
Last Version Date
2024-11-20
Entry Detail
PDB ID:
5OH0
Keywords:
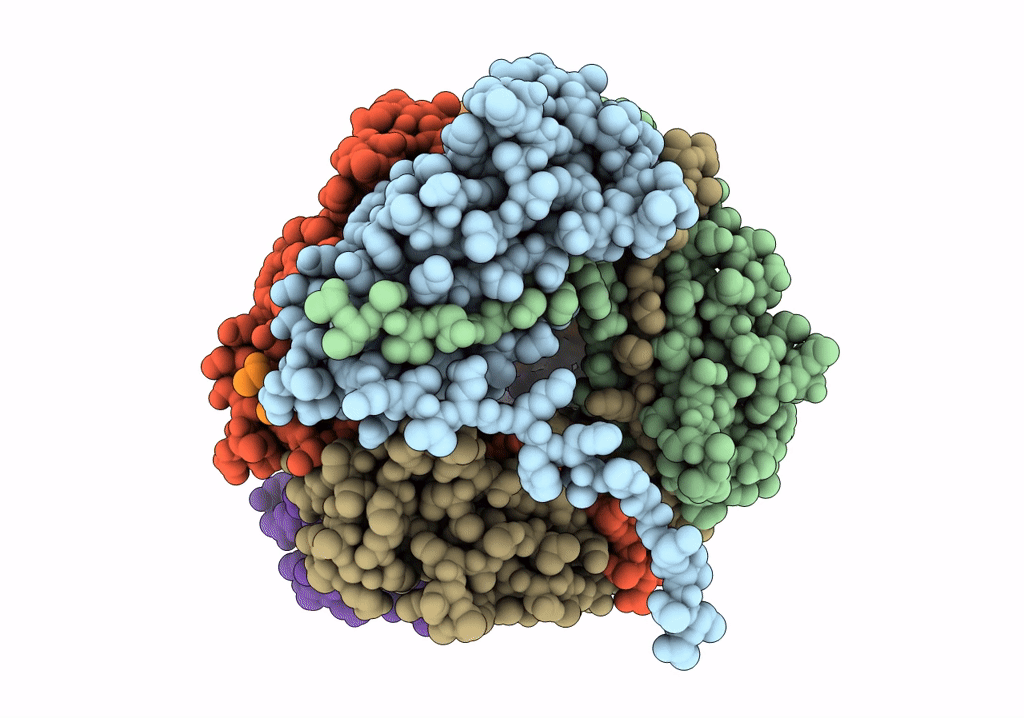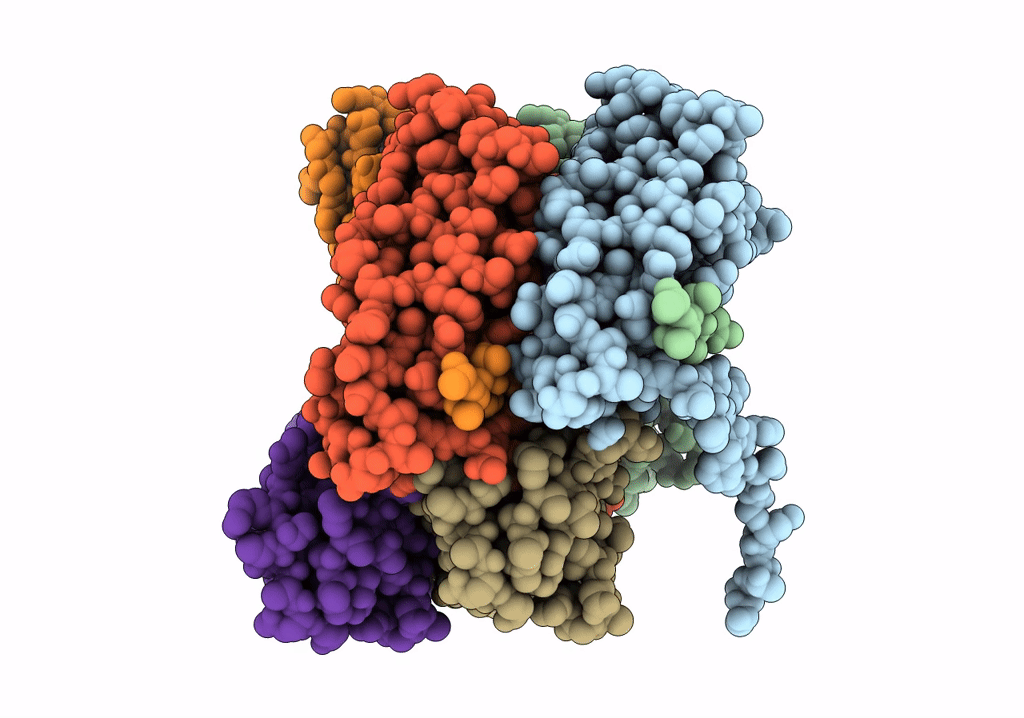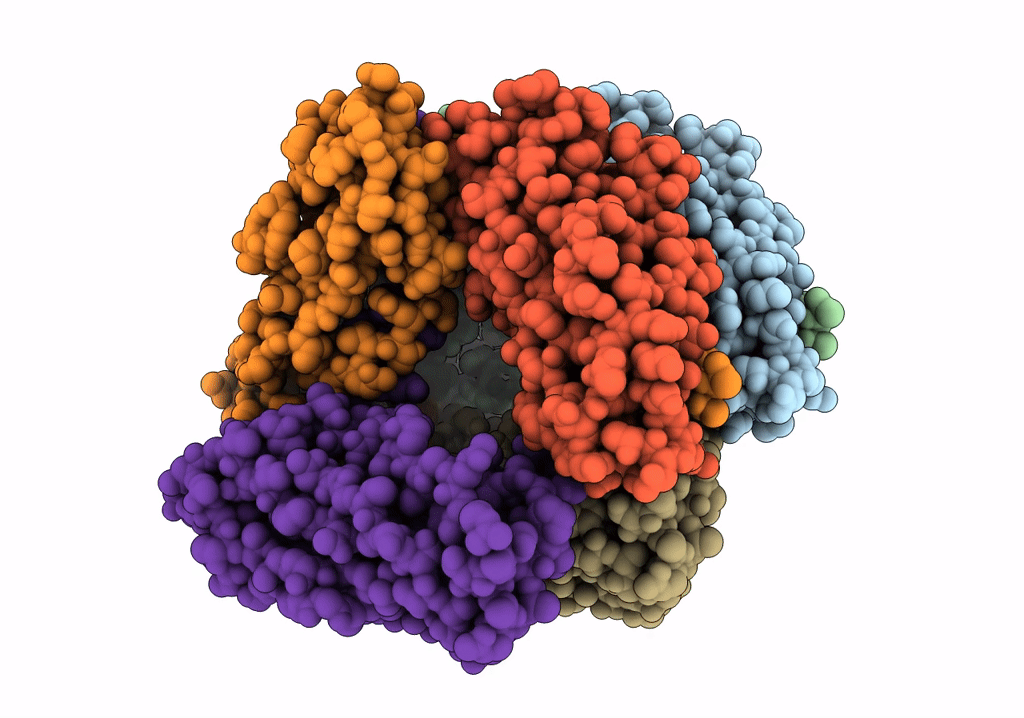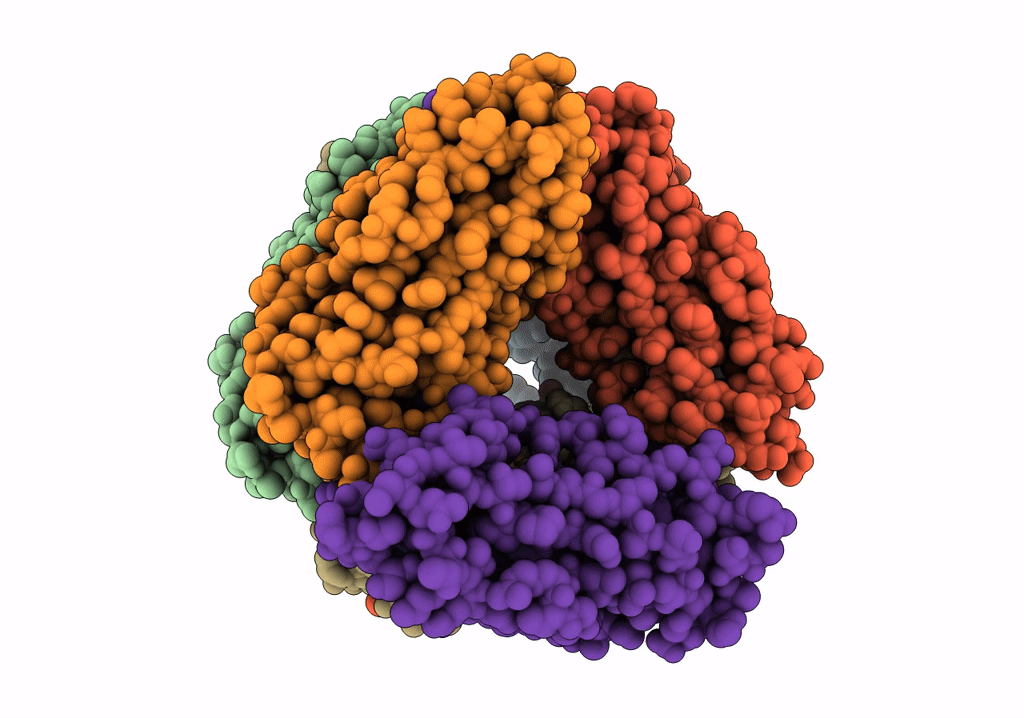
Title:
The Cryo-Electron Microscopy Structure of the Type 1 Chaperone-Usher Pilus Rod
Biological Source:
Source Organism:
Escherichia coli J96 (Taxon ID: 1206108)
Host Organism:
Method Details:
Experimental Method:
Resolution:
4.20 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
HELICAL


