Deposition Date
2017-07-13
Release Date
2017-10-25
Last Version Date
2024-01-17
Entry Detail
PDB ID:
5OGL
Keywords:
Title:
Structure of bacterial oligosaccharyltransferase PglB in complex with an acceptor peptide and an lipid-linked oligosaccharide analog
Biological Source:
Source Organism(s):
Campylobacter lari (strain RM2100 / D67 / ATCC BAA-1060) (Taxon ID: 306263)
Campylobacter jejuni (Taxon ID: 197)
Campylobacter jejuni (Taxon ID: 197)
Expression System(s):
Method Details:
Experimental Method:
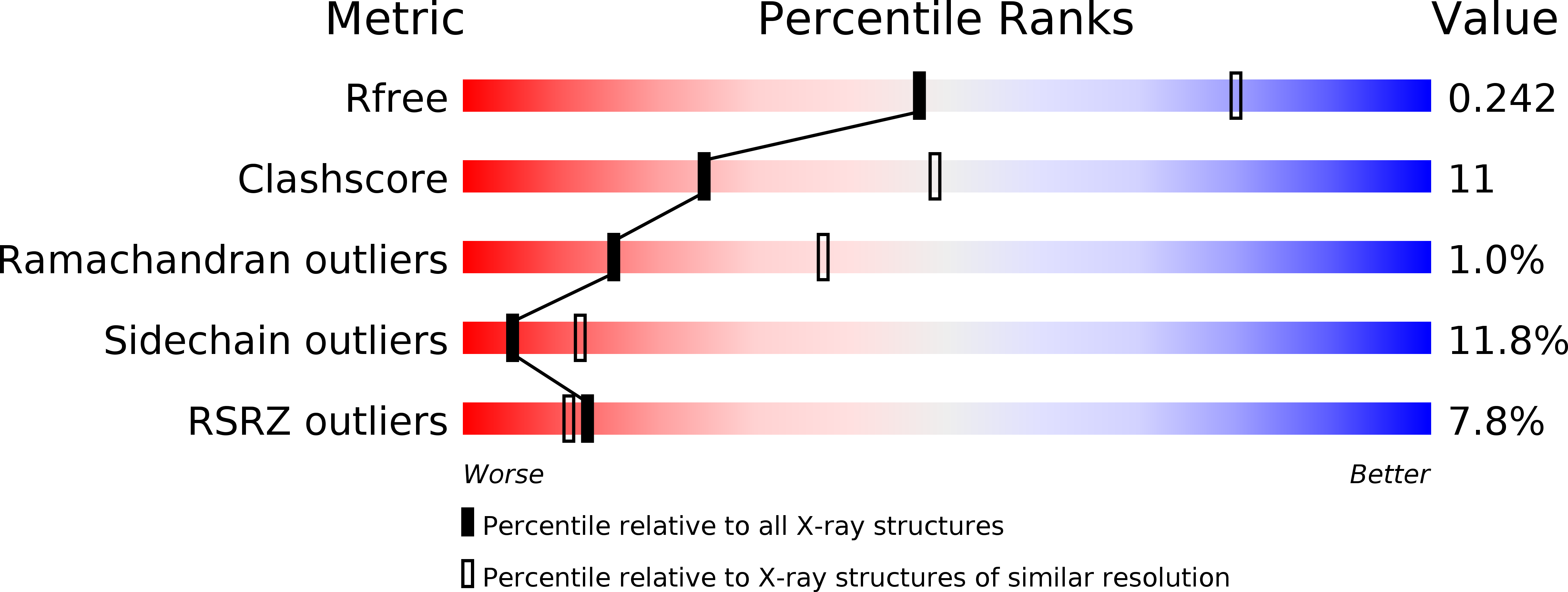
Resolution:
2.70 Å
R-Value Free:
0.24
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 21 21 21