Deposition Date
2017-07-11
Release Date
2017-11-29
Last Version Date
2024-01-17
Entry Detail
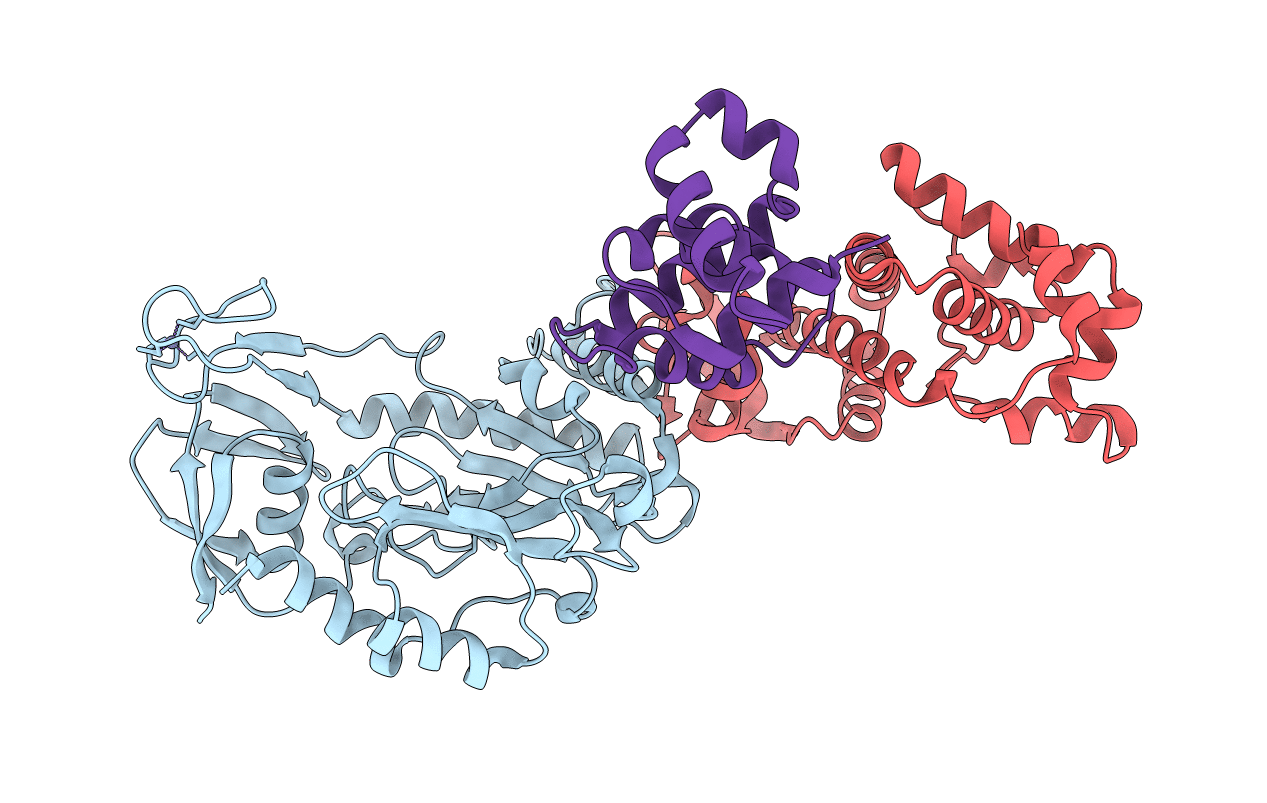
PDB ID:
5OFN
Keywords:
Title:
Crystal structure of the heterotrimeric PriSLX primase from S. solfataricus.
Biological Source:
Source Organism(s):
Sulfolobus solfataricus (strain ATCC 35092 / DSM 1617 / JCM 11322 / P2) (Taxon ID: 273057)
Sulfolobus solfataricus (Taxon ID: 2287)
Sulfolobus solfataricus (Taxon ID: 2287)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.01 Å
R-Value Free:
0.29
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
C 1 2 1


