Deposition Date
2017-07-05
Release Date
2017-08-30
Last Version Date
2024-11-13
Entry Detail
PDB ID:
5ODC
Keywords:
Title:
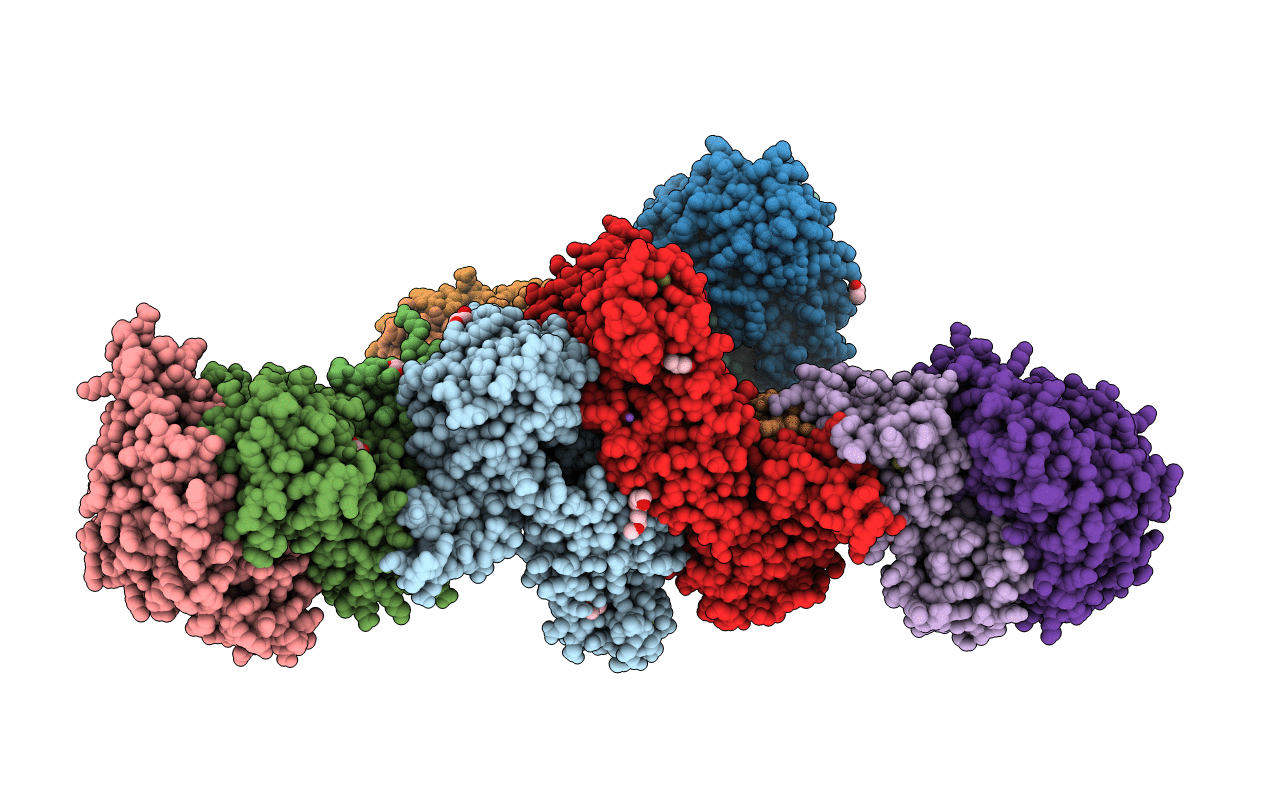
Heterodisulfide reductase / [NiFe]-hydrogenase complex from Methanothermococcus thermolithotrophicus at 2.3 A resolution
Biological Source:
Source Organism(s):
Method Details:
Experimental Method:
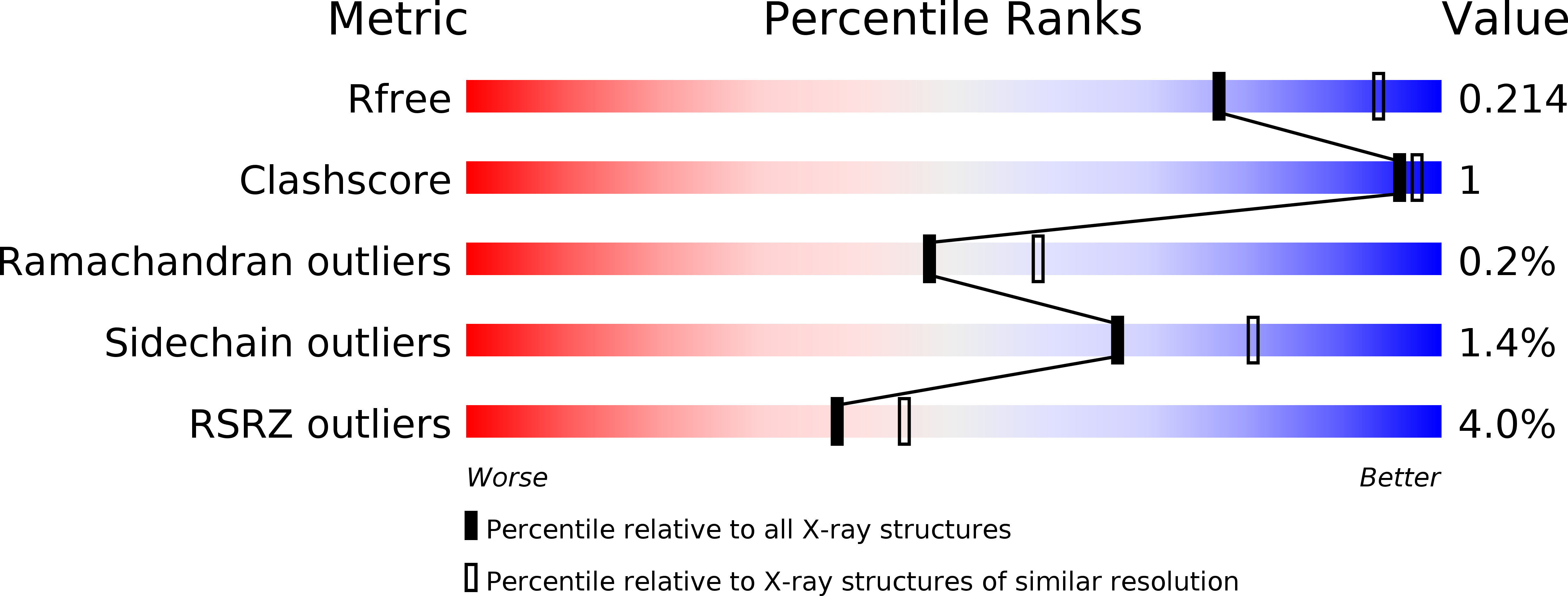
Resolution:
2.30 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1