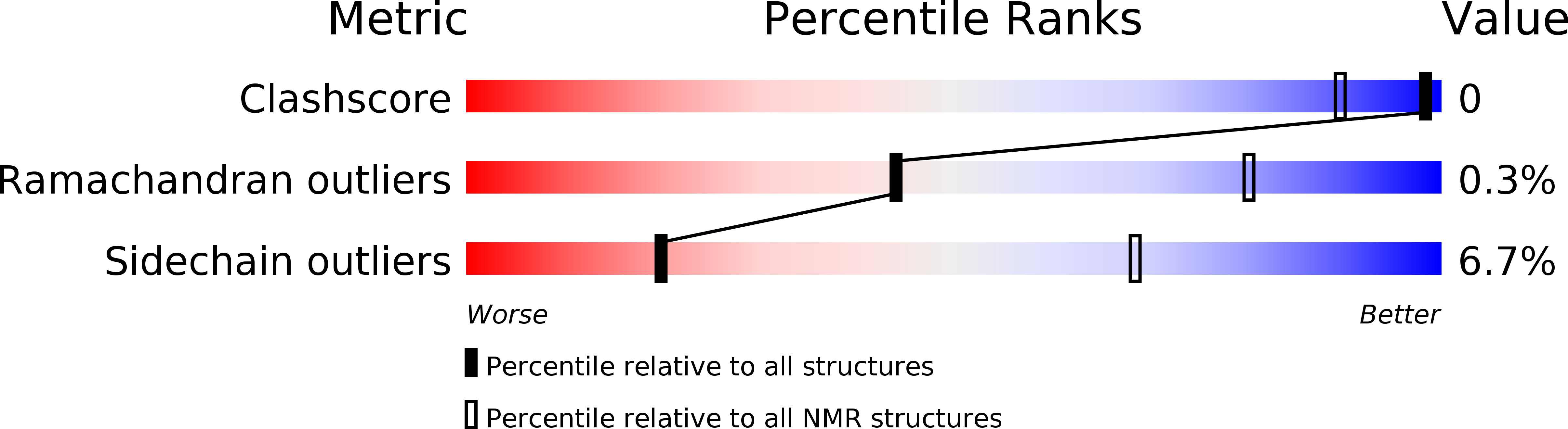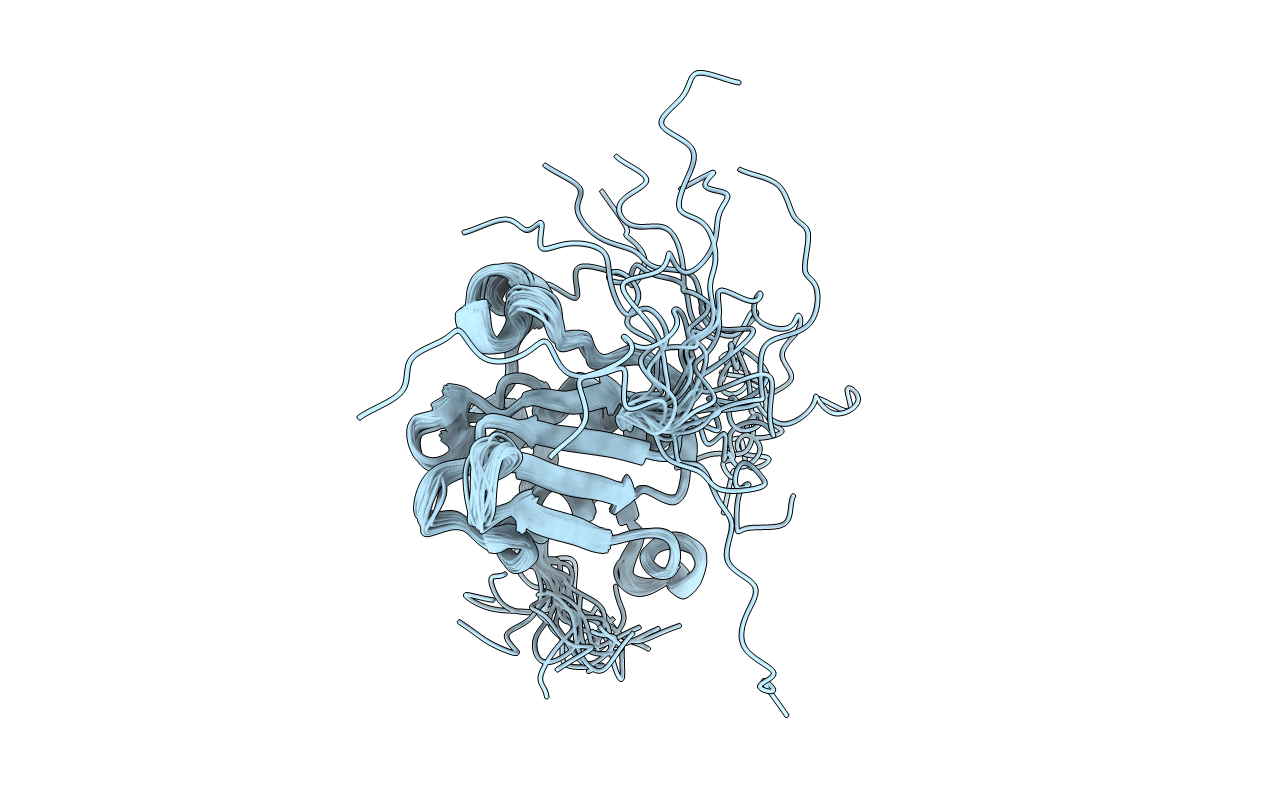
Deposition Date
2017-06-28
Release Date
2018-01-24
Last Version Date
2024-06-19
Entry Detail
PDB ID:
5OBN
Keywords:
Title:
NMR solution structure of U11/U12 65K protein's C-terminal RRM domain (381-516)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
20
Selection Criteria:
target function