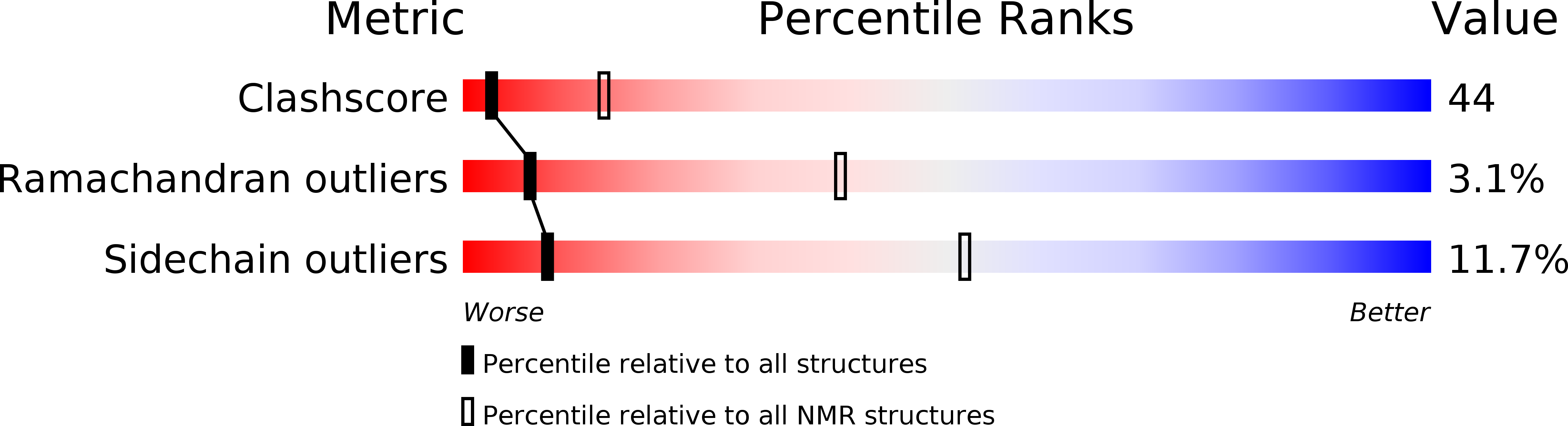Deposition Date
2017-06-25
Release Date
2018-01-03
Last Version Date
2024-05-15
Entry Detail
PDB ID:
5OAY
Keywords:
Title:
M. tuberculosis [4Fe-4S] protein WhiB1 is a four-helix bundle that forms a NO-sensitive complex with sigmaA and regulates the major virulence factor ESX-1
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
62
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy