Deposition Date
2017-06-05
Release Date
2017-08-23
Last Version Date
2024-05-01
Entry Detail
PDB ID:
5O68
Keywords:
Title:
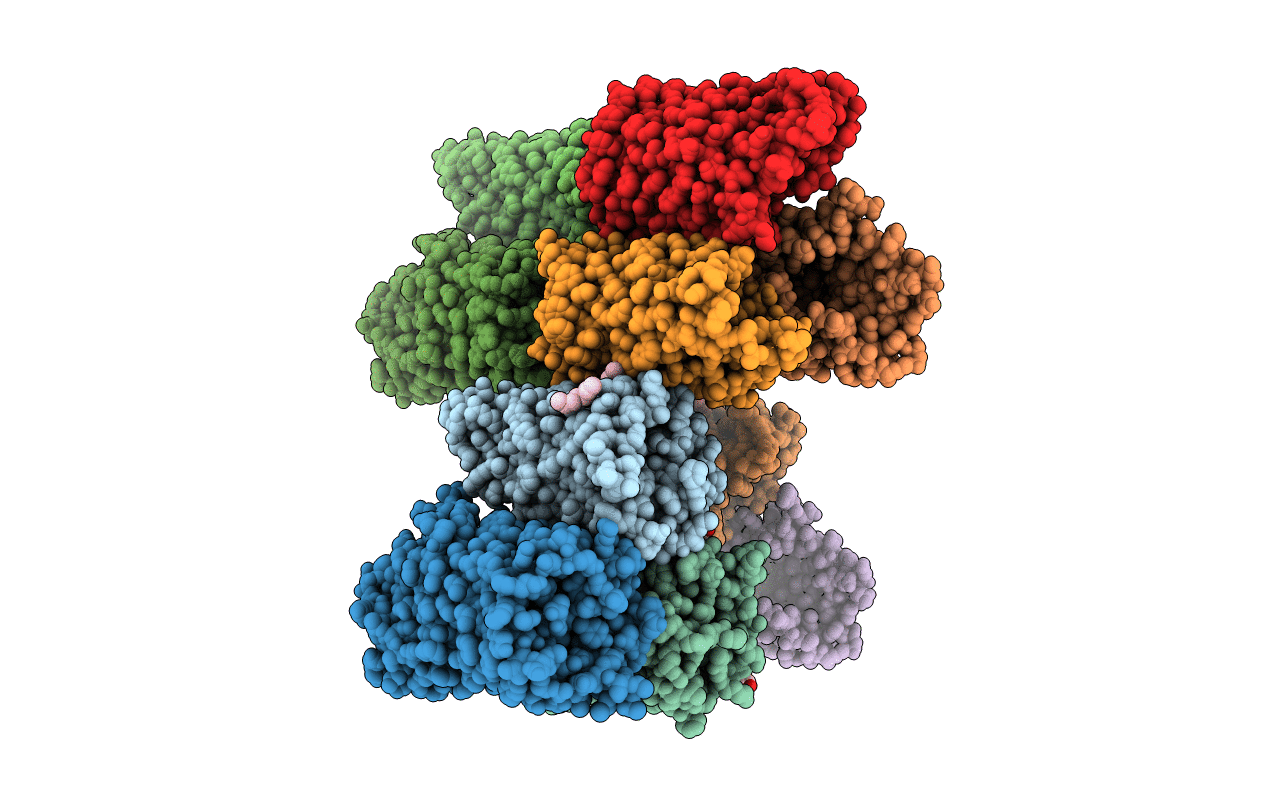
Crystal Structure of the Pseudomonas functional amyloid secretion protein FapF - R157A mutant
Biological Source:
Source Organism(s):
Pseudomonas sp. UK4 (Taxon ID: 452680)
Expression System(s):
Method Details:
Experimental Method:
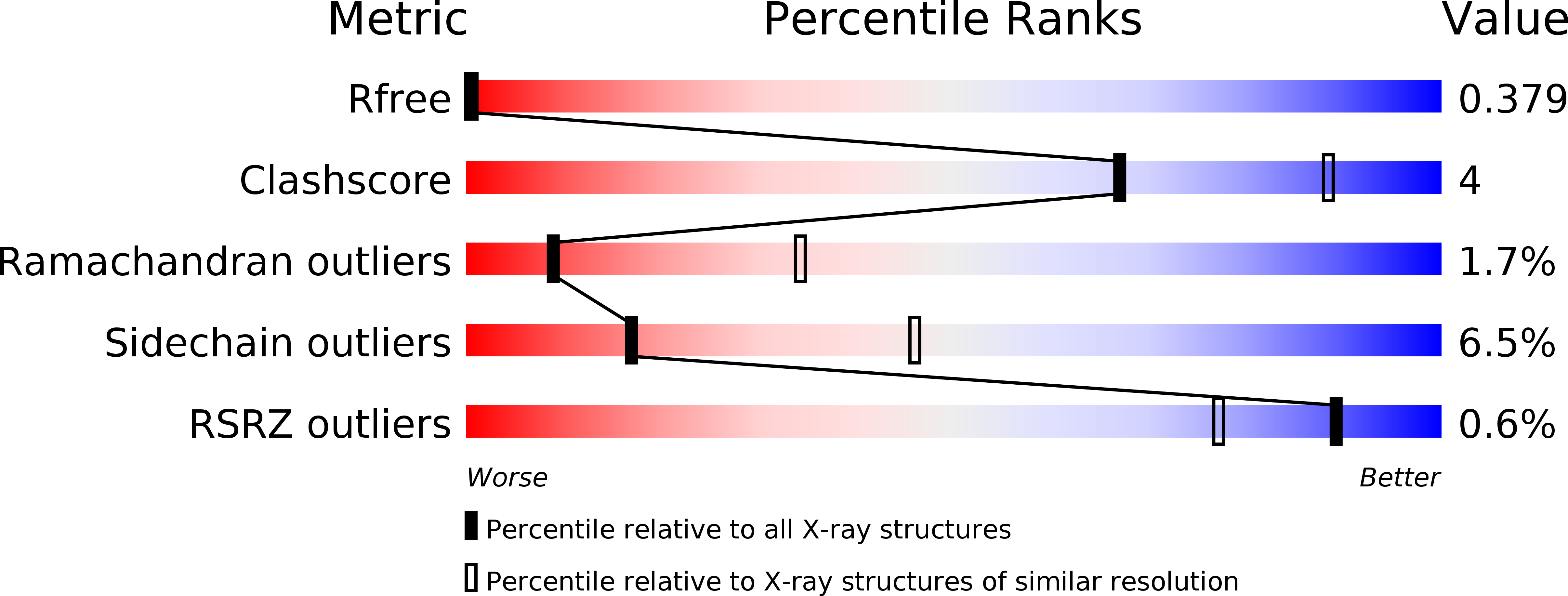
Resolution:
3.08 Å
R-Value Free:
0.36
R-Value Work:
0.31
R-Value Observed:
0.32
Space Group:
P 1