Deposition Date
2017-05-08
Release Date
2017-12-06
Last Version Date
2024-11-13
Entry Detail
PDB ID:
5NWU
Keywords:
Title:
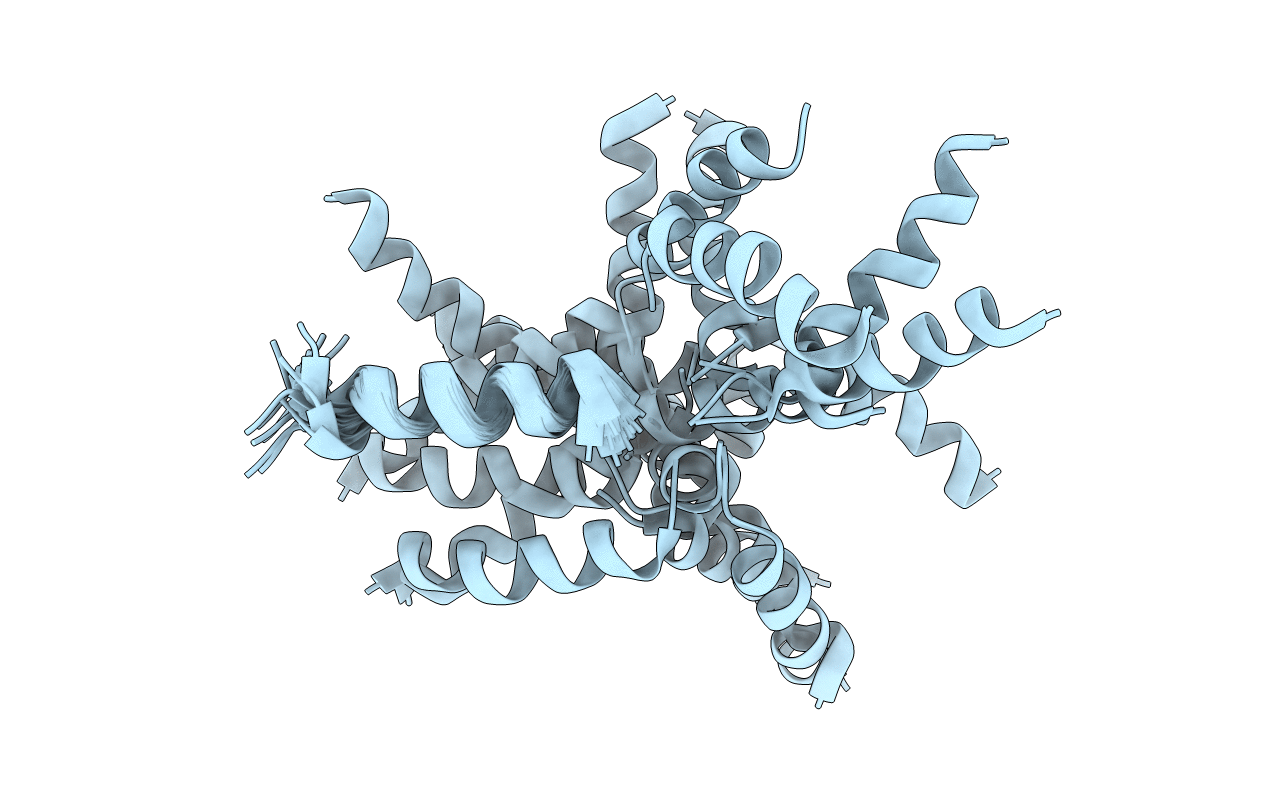
NMR assignment and structure of a peptide derived from the fusion peptide of HIV-1 gp41 in the presence of hexafluoroisopropanol
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
20
Selection Criteria:
structures with the least restraint violations


