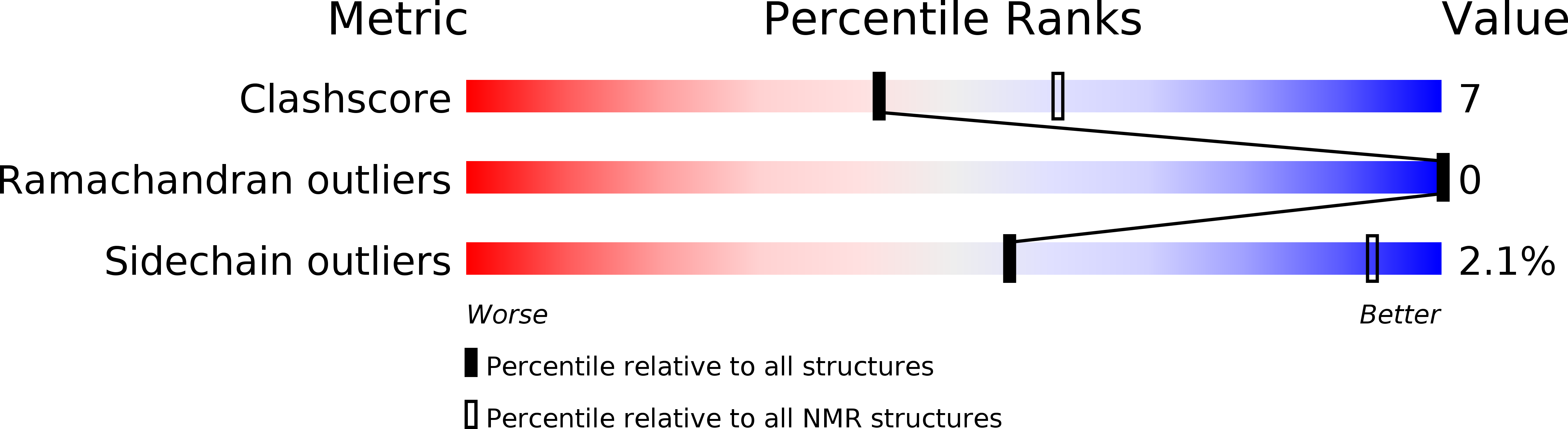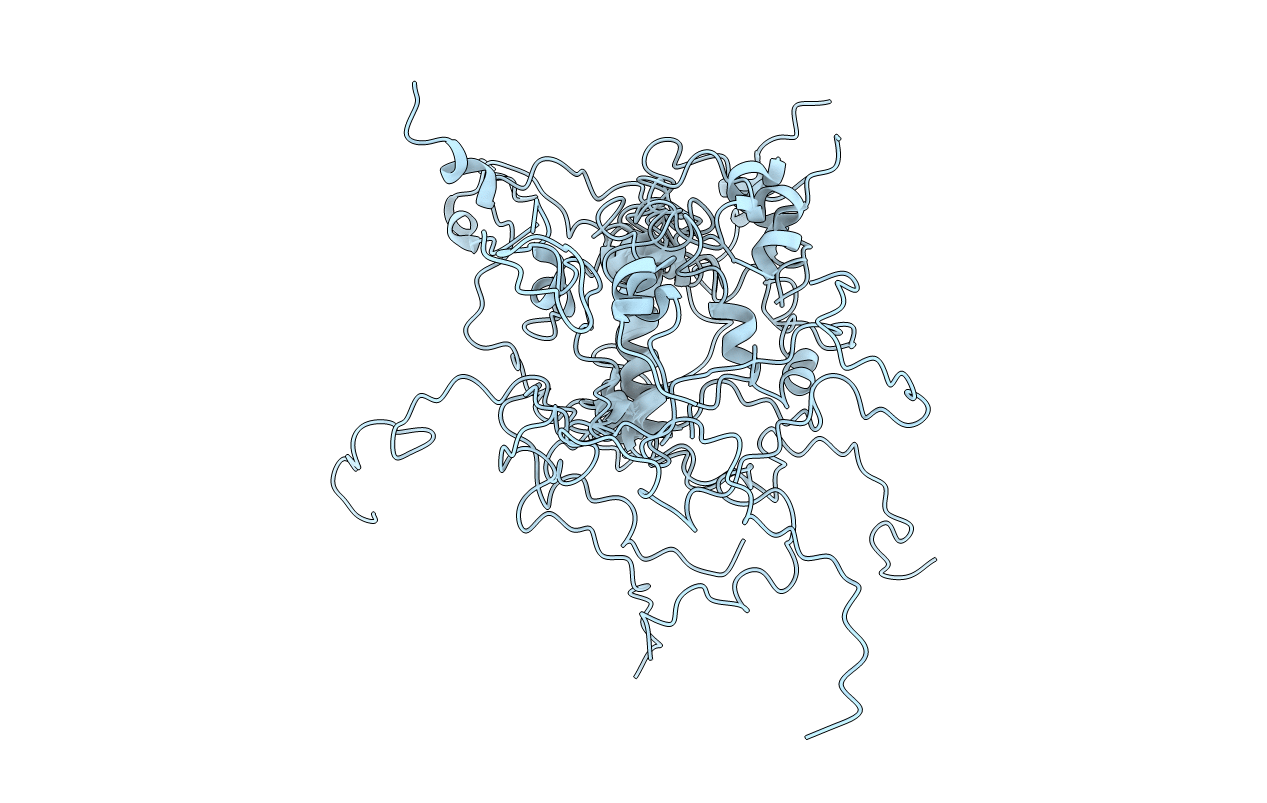
Deposition Date
2017-03-22
Release Date
2017-04-26
Last Version Date
2024-06-19
Entry Detail
PDB ID:
5NHQ
Keywords:
Title:
Nuclear Magnetic Resonance Structure of the Human Polyoma JC Virus Agnoprotein
Biological Source:
Source Organism(s):
JC polyomavirus (Taxon ID: 10632)
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
12
Selection Criteria:
structures with the lowest energy