Deposition Date
2017-03-02
Release Date
2018-01-10
Last Version Date
2024-11-06
Entry Detail
PDB ID:
5NC0
Keywords:
Title:
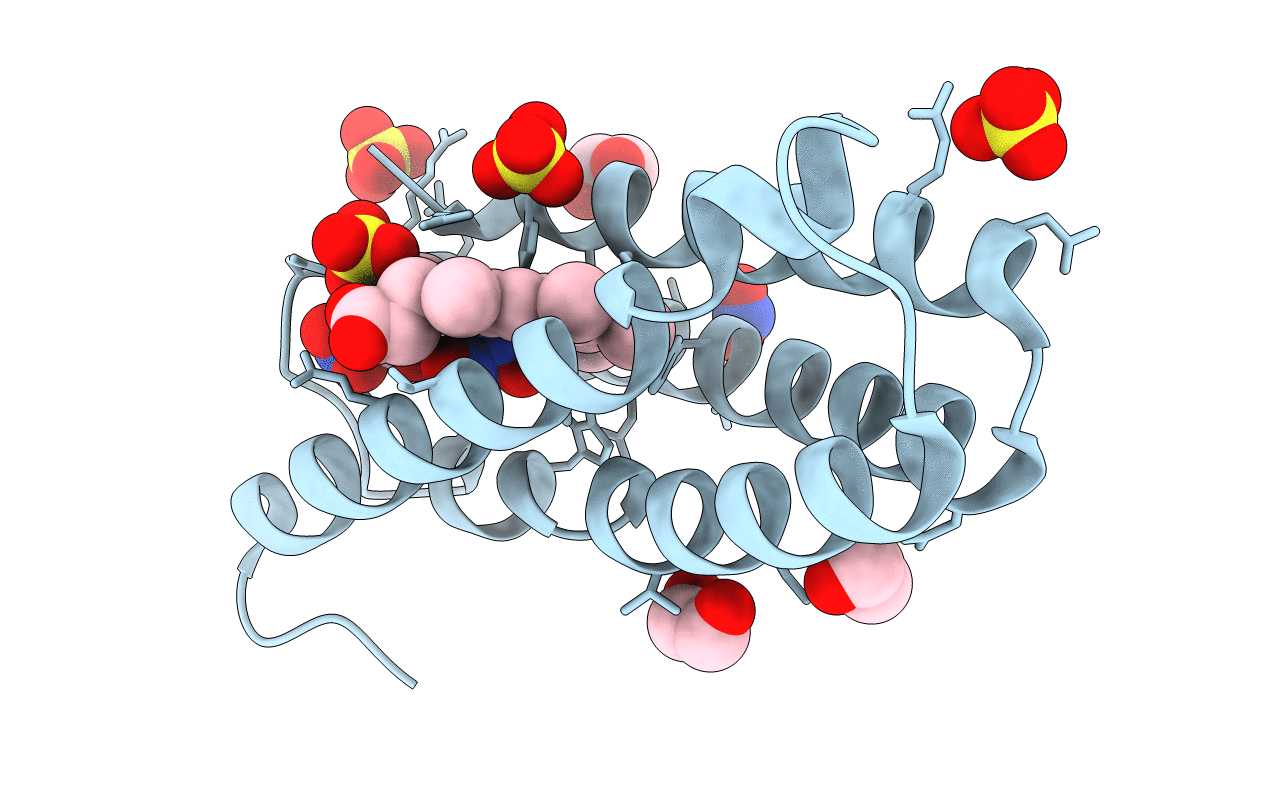
The 0.91 A resolution structure of the L16G mutant of cytochrome c prime from Alcaligenes xylosoxidans, complexed with nitric oxide
Biological Source:
Source Organism:
Alcaligenes xylosoxydans xylosoxydans (Taxon ID: 85698)
Host Organism:
Method Details:
Experimental Method:
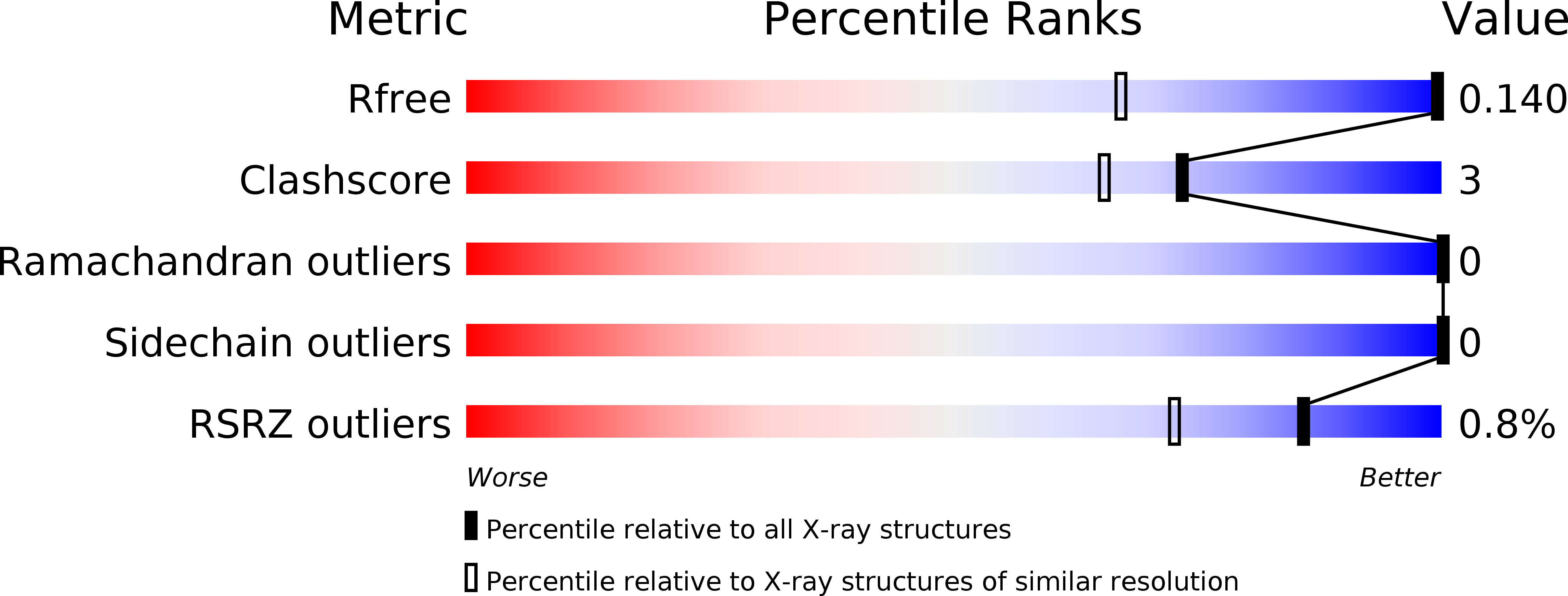
Resolution:
0.91 Å
R-Value Free:
0.13
R-Value Work:
0.12
R-Value Observed:
0.12
Space Group:
P 65 2 2