Deposition Date
2017-03-02
Release Date
2018-08-22
Last Version Date
2024-01-17
Entry Detail
PDB ID:
5NBN
Keywords:
Title:
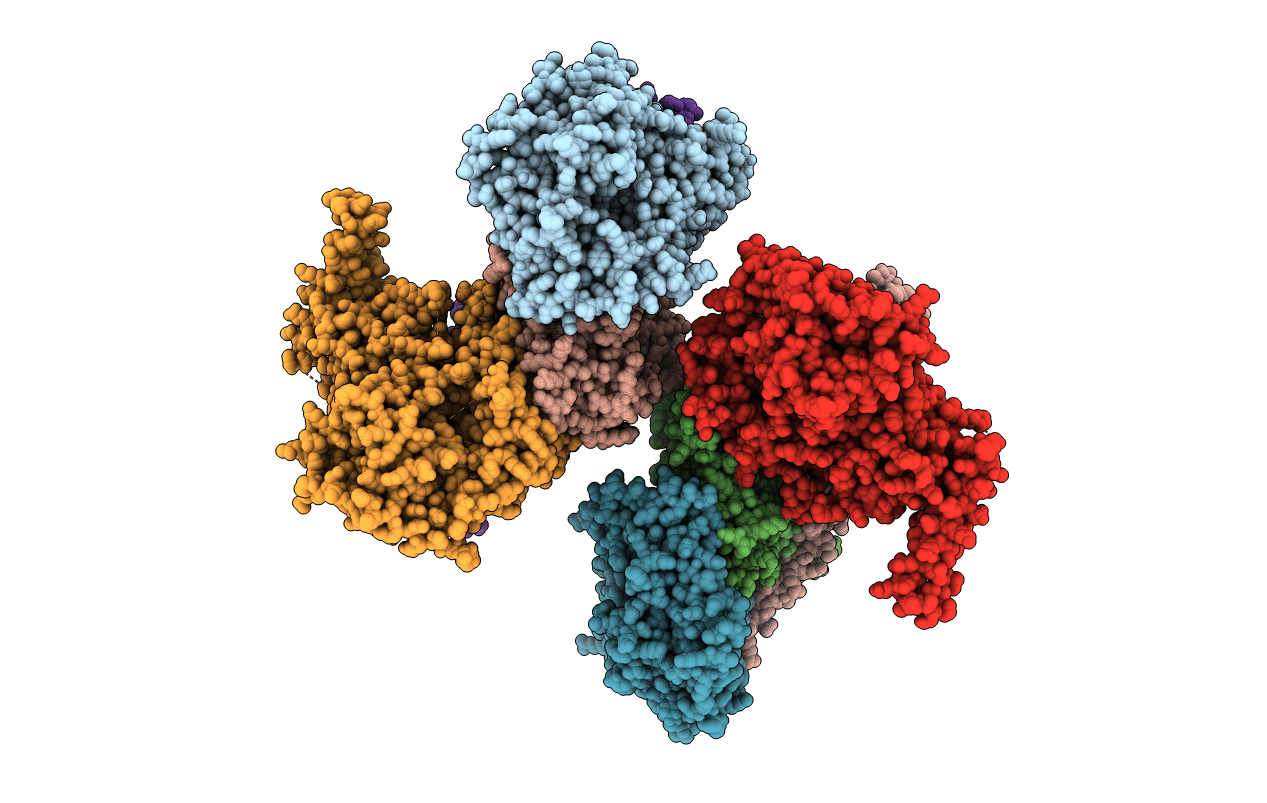
Crystal structure of the Arp4-N-actin-Arp8-Ino80HSA module of INO80
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
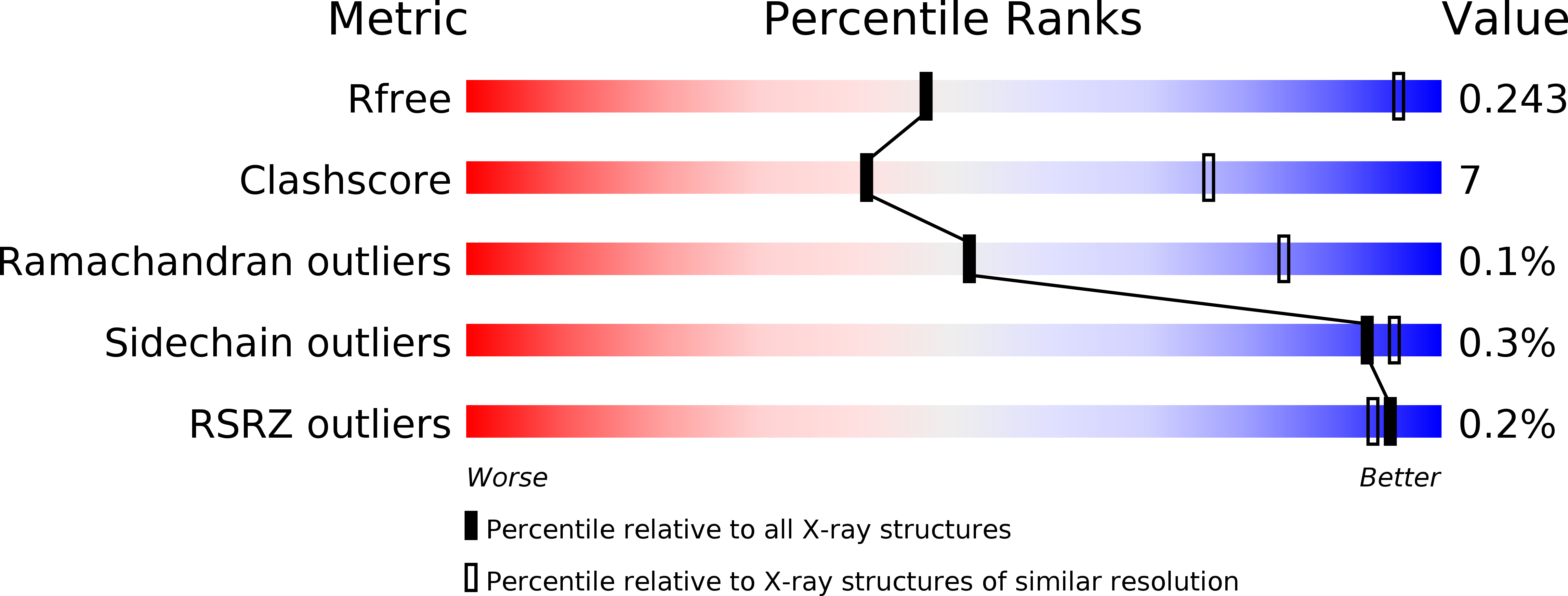
Resolution:
4.00 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 2 2 21