Deposition Date
2017-02-24
Release Date
2018-02-21
Last Version Date
2024-05-01
Entry Detail
PDB ID:
5N9I
Keywords:
Title:
STRUCTURE OF 206-GVVTSE-211, THE STERIC ZIPPER THAT SUPPORTS THE SELF-ASSOCIATION OF P. STUARTII OMP-PST1 INTO DIMERS OF TRIMERS
Biological Source:
Source Organism:
Providencia stuartii (Taxon ID: 588)
Method Details:
Experimental Method:
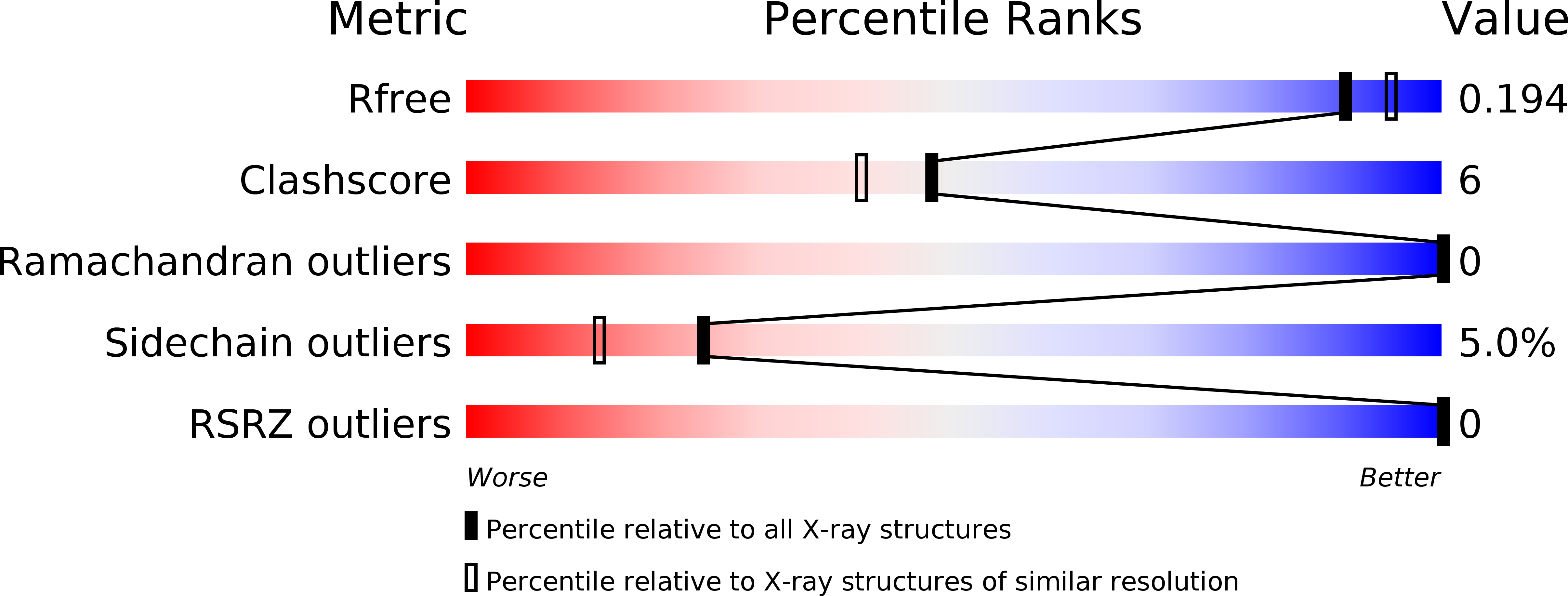
Resolution:
1.91 Å
R-Value Free:
0.20
R-Value Work:
0.14
R-Value Observed:
0.15
Space Group:
P 1