Deposition Date
2016-12-08
Release Date
2017-03-29
Last Version Date
2024-01-17
Entry Detail
PDB ID:
5MM9
Keywords:
Title:
VIM-2_2b. Metallo-beta-Lactamase Inhibitors by Bioisosteric Replacement: Preparation, Activity and Binding
Biological Source:
Source Organism(s):
Pseudomonas aeruginosa (Taxon ID: 287)
Expression System(s):
Method Details:
Experimental Method:
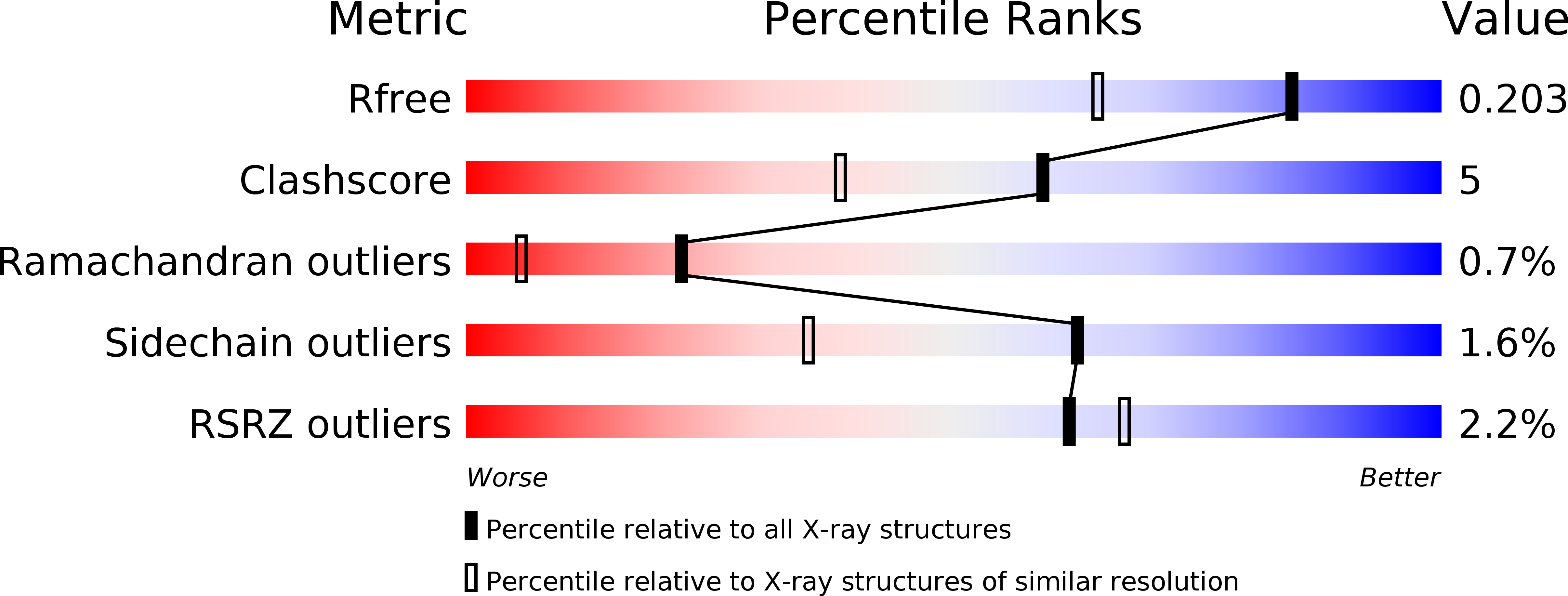
Resolution:
1.55 Å
R-Value Free:
0.20
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
C 1 2 1