Deposition Date
2016-12-05
Release Date
2017-06-14
Last Version Date
2024-01-17
Entry Detail
PDB ID:
5MKP
Keywords:
Title:
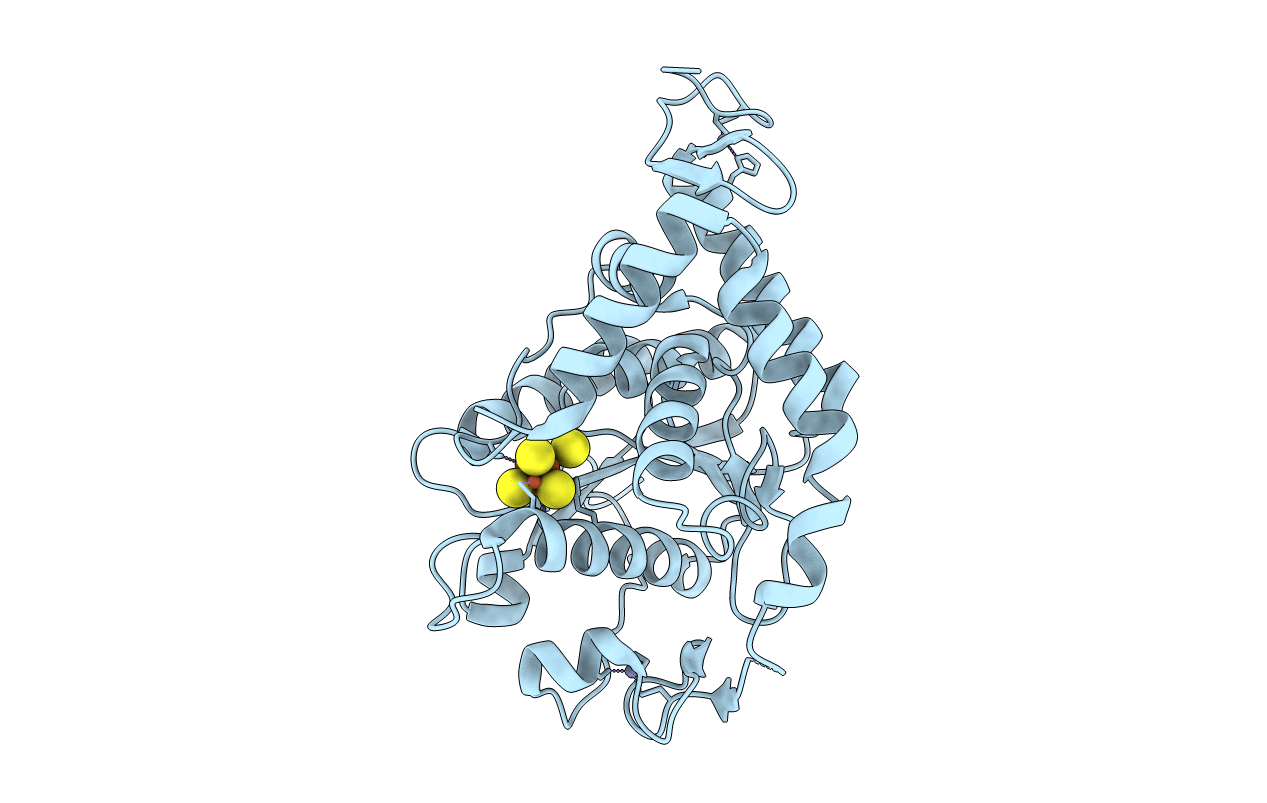
Non redox thiolation in transfer RNAs occuring via sulfur activation by a [4Fe-4S] cluster
Biological Source:
Source Organism(s):
Pyrococcus horikoshii OT3 (Taxon ID: 70601)
Expression System(s):
Method Details:
Experimental Method:
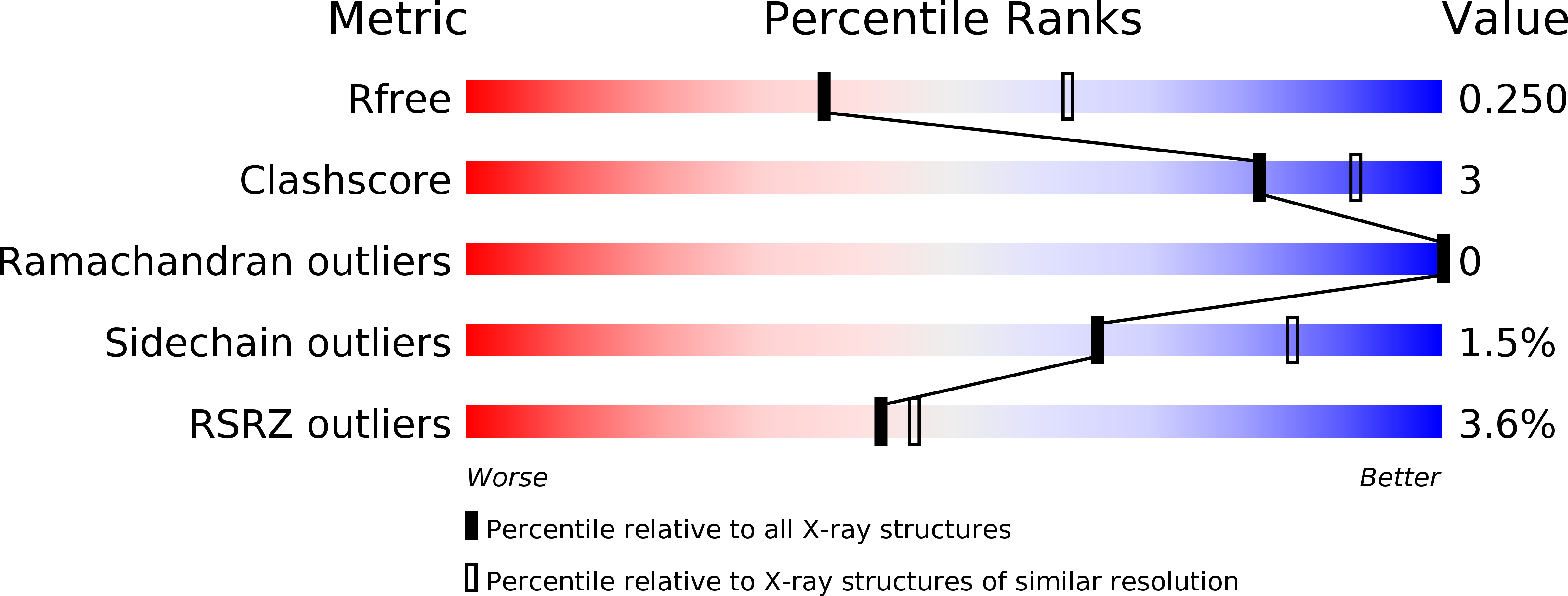
Resolution:
2.50 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 43 21 2