Deposition Date
2016-11-22
Release Date
2016-12-14
Last Version Date
2024-11-13
Entry Detail
PDB ID:
5MGY
Keywords:
Title:
Crystal structure of Pseudomonas stutzeri flavinyl transferase ApbE, apo form
Biological Source:
Source Organism:
Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (Taxon ID: 32042)
Host Organism:
Method Details:
Experimental Method:
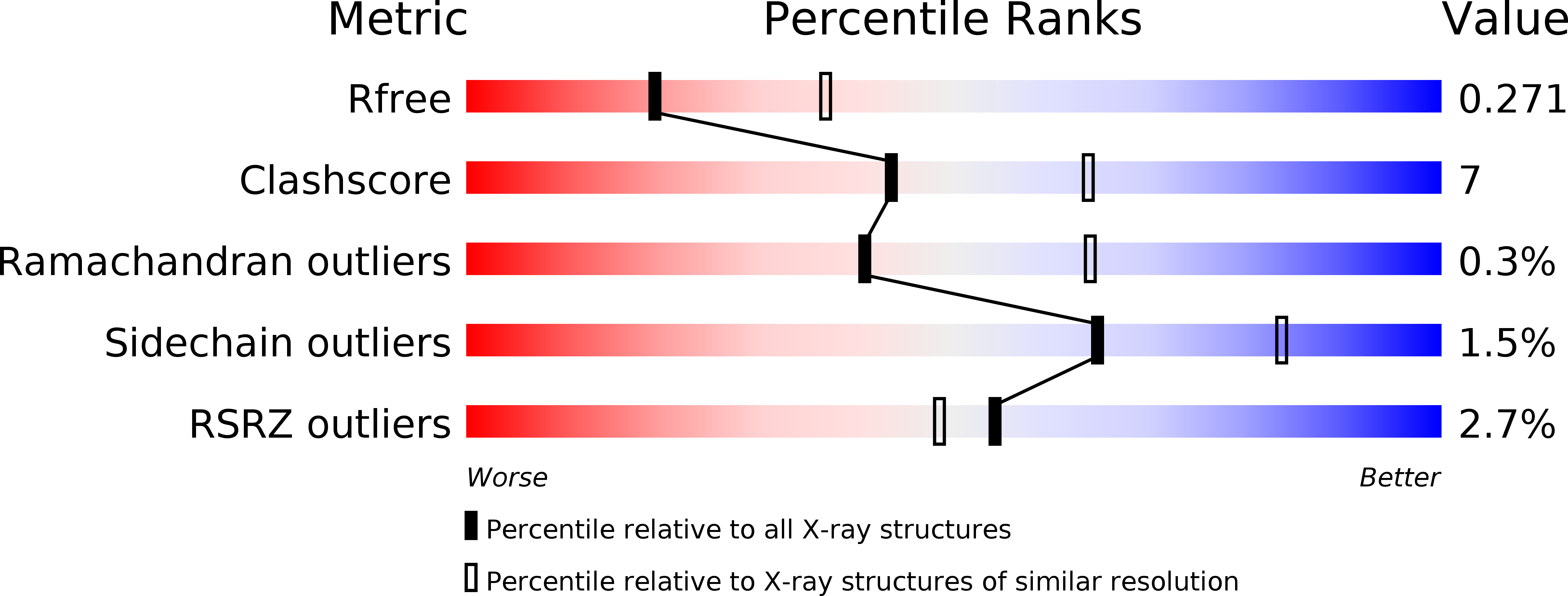
Resolution:
2.60 Å
R-Value Free:
0.27
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 1 21 1