Deposition Date
2016-11-20
Release Date
2017-12-20
Last Version Date
2024-10-09
Entry Detail
PDB ID:
5MG5
Keywords:
Title:
A multi-component acyltransferase PhlABC from Pseudomonas protegens soaked with the monoacetylphloroglucinol (MAPG)
Biological Source:
Source Organism(s):
Pseudomonas protegens (Taxon ID: 380021)
Pseudomonas fluorescens (strain ATCC BAA-477 / NRRL B-23932 / Pf-5) (Taxon ID: 220664)
Pseudomonas fluorescens (strain ATCC BAA-477 / NRRL B-23932 / Pf-5) (Taxon ID: 220664)
Expression System(s):
Method Details:
Experimental Method:
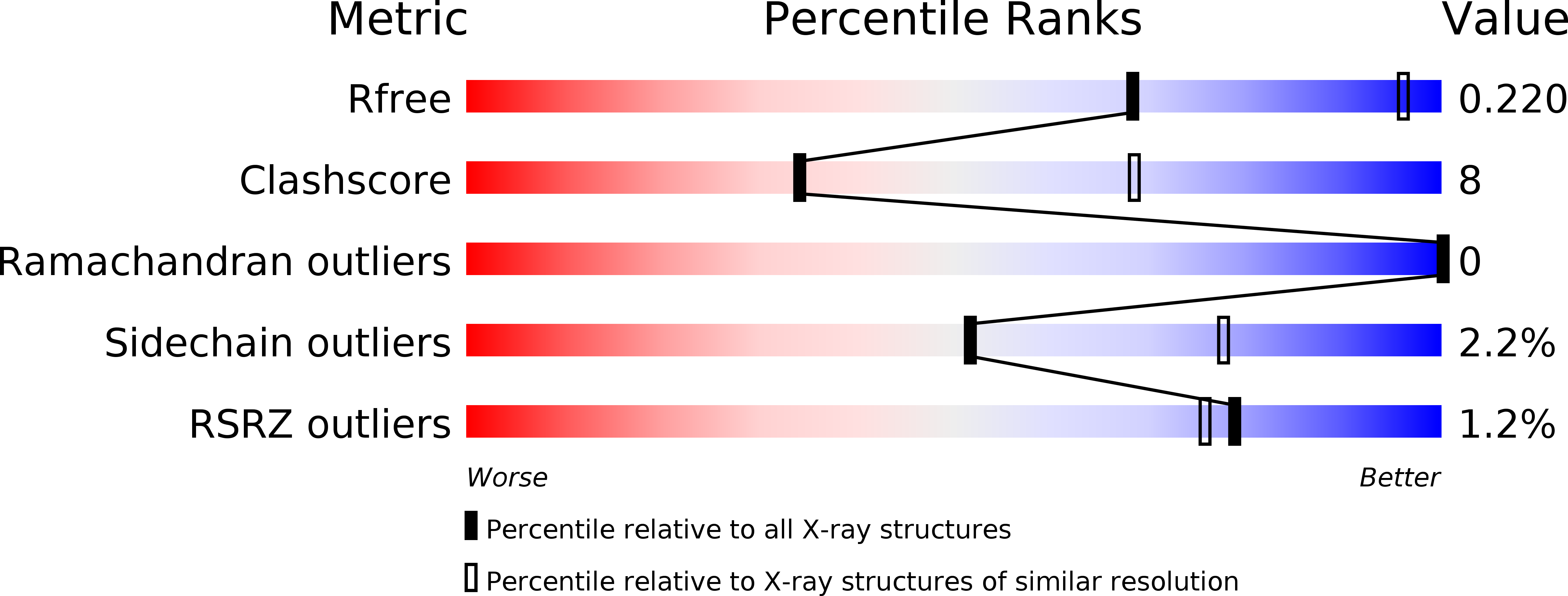
Resolution:
3.44 Å
R-Value Free:
0.22
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 21 21 21