Deposition Date
2016-11-18
Release Date
2017-07-19
Last Version Date
2024-01-17
Entry Detail
PDB ID:
5MFD
Keywords:
Title:
Designed armadillo repeat protein YIIIM''6AII in complex with pD_(KR)5
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Enterobacteria phage lambda (Taxon ID: 10710)
Enterobacteria phage lambda (Taxon ID: 10710)
Expression System(s):
Method Details:
Experimental Method:
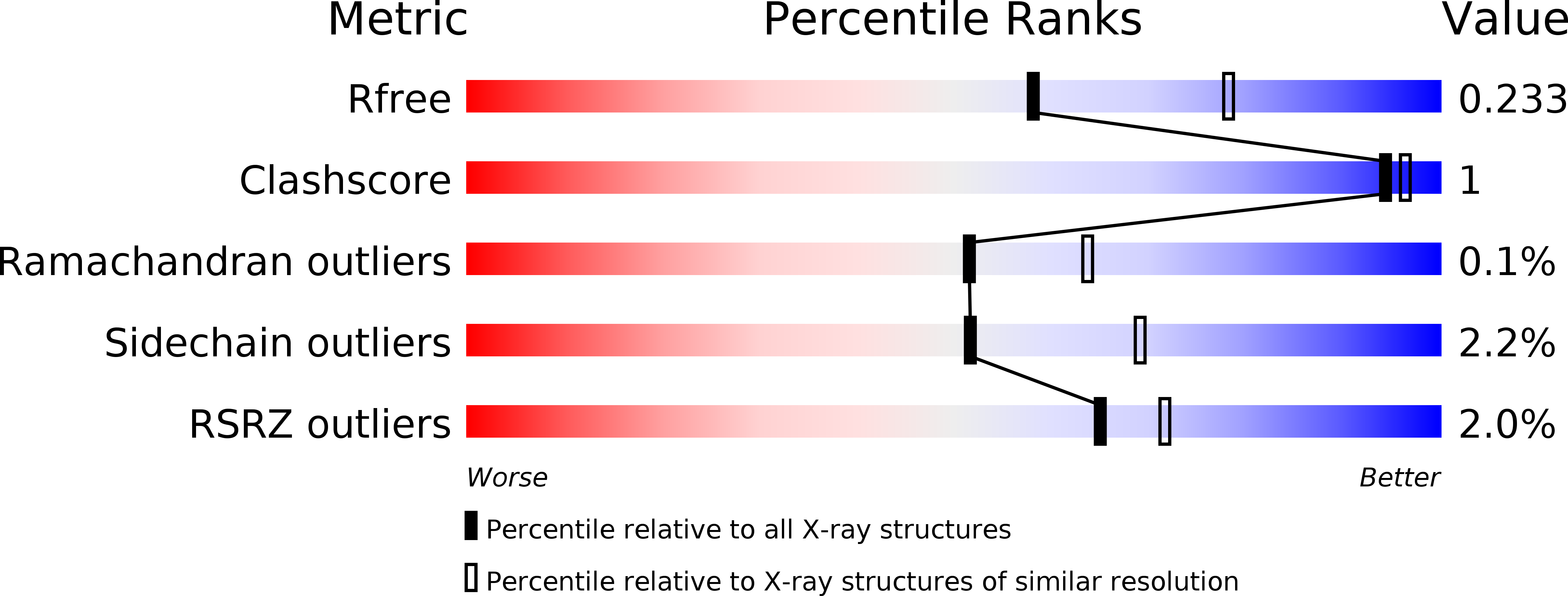
Resolution:
2.30 Å
R-Value Free:
0.21
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 63