Deposition Date
2016-11-16
Release Date
2017-05-10
Last Version Date
2024-10-16
Entry Detail
PDB ID:
5MEW
Keywords:
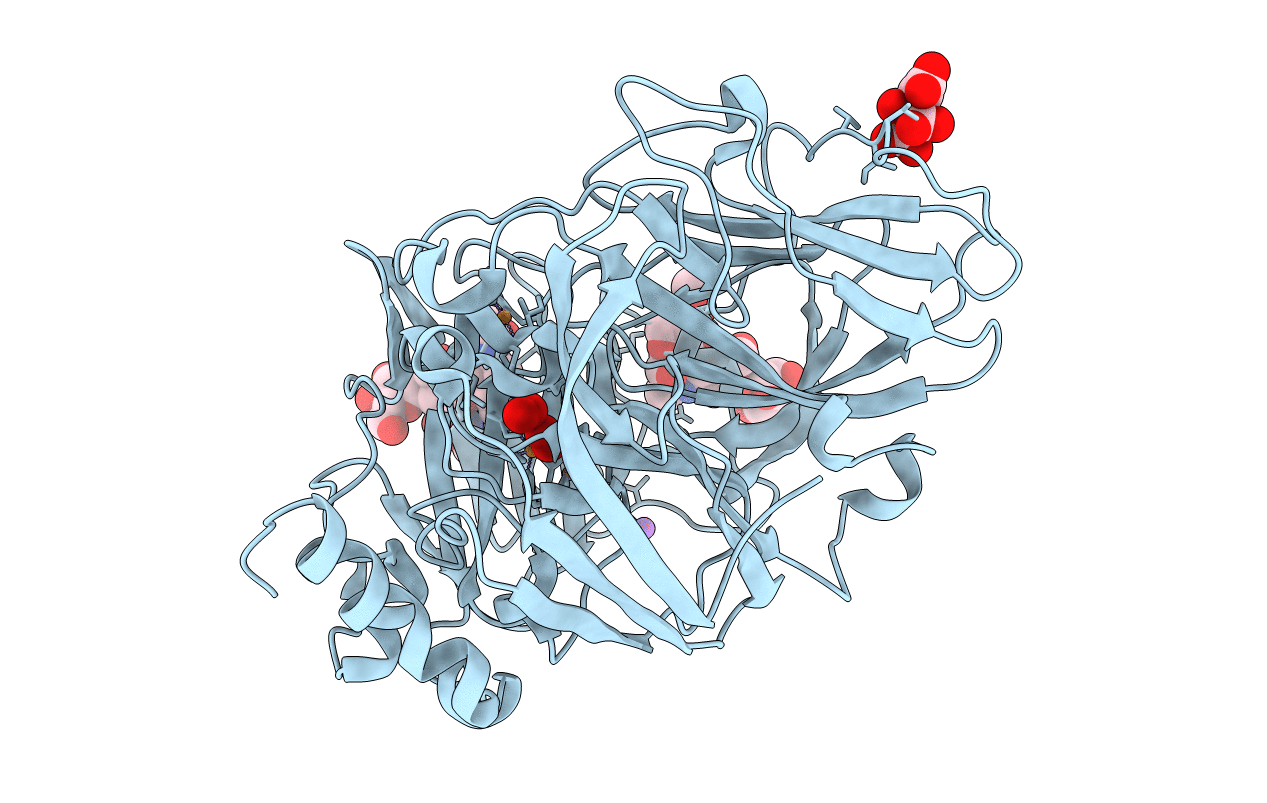
Title:
The study of the X-ray induced enzymatic reduction of molecular oxygen to water for laccase from Steccherinum murashkinskyi. Second structure of the series with total exposition time 33 min.
Biological Source:
Source Organism(s):
Steccherinum murashkinskyi (Taxon ID: 627145)
Method Details:
Experimental Method:
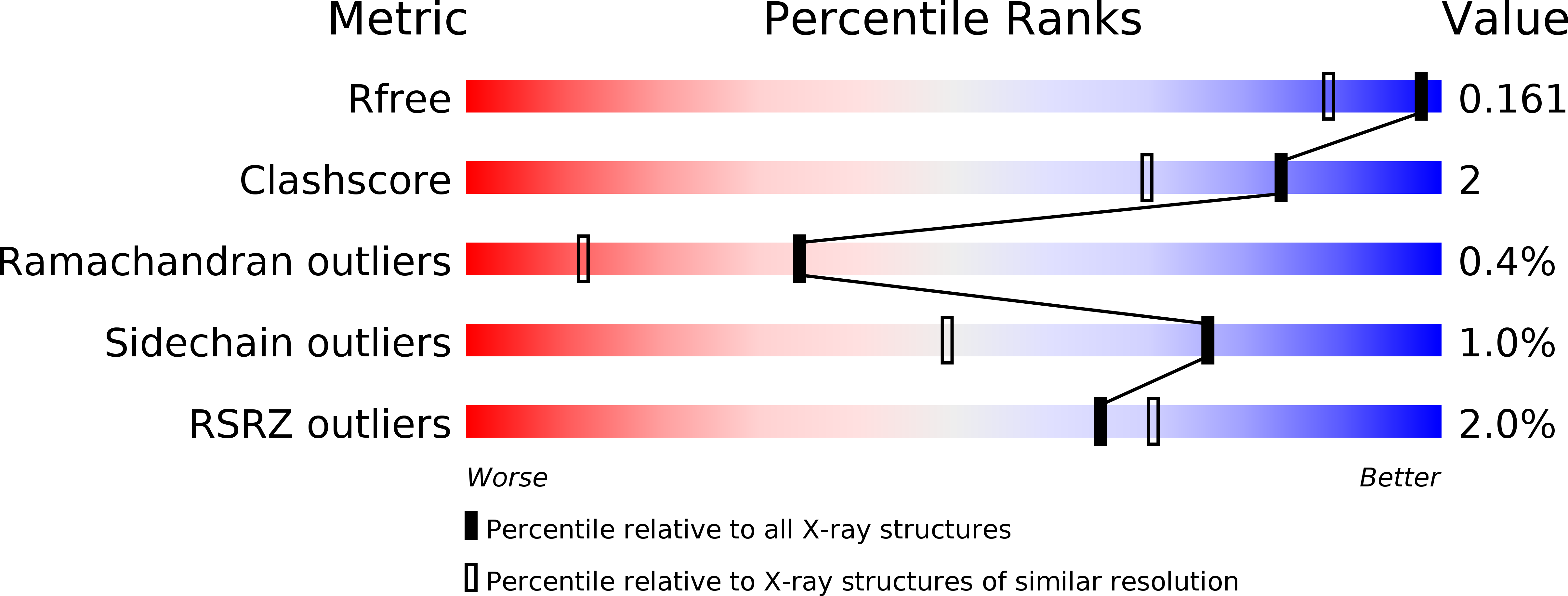
Resolution:
1.35 Å
R-Value Free:
0.15
R-Value Work:
0.13
R-Value Observed:
0.13
Space Group:
P 21 21 21