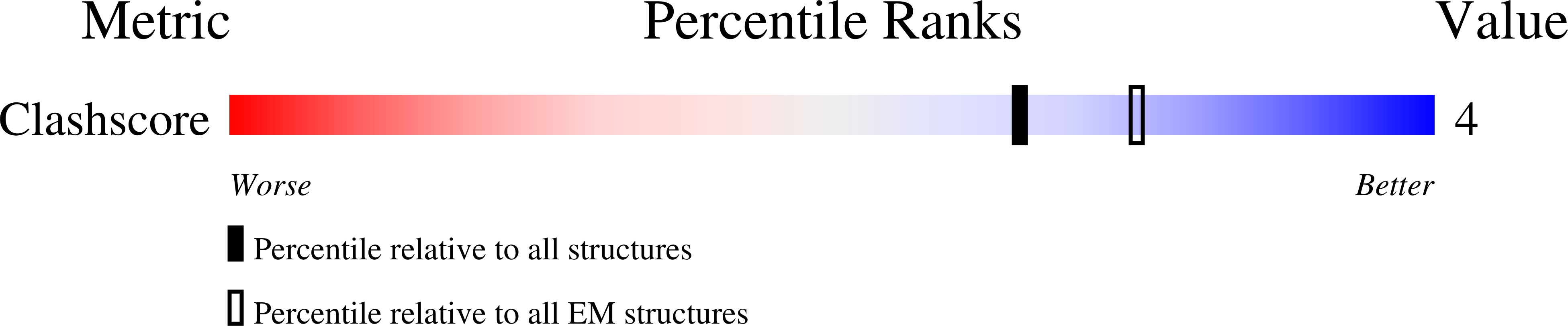Deposition Date
2016-10-22
Release Date
2016-11-30
Last Version Date
2024-05-08
Entry Detail
PDB ID:
5M5O
Keywords:
Title:
Pseudo-atomic model of microtubule-bound S.pombe kinesin-5 motor domain in the AMPPNP state (based on cryo-electron microscopy experiment): the N-terminus adopts multiple conformations.
Biological Source:
Source Organism(s):
Schizosaccharomyces pombe (strain 972 / ATCC 24843) (Taxon ID: 284812)
Bos taurus (Taxon ID: 9913)
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
9.30 Å
Aggregation State:
HELICAL ARRAY
Reconstruction Method:
SINGLE PARTICLE