Deposition Date
2016-09-29
Release Date
2016-11-23
Last Version Date
2024-10-16
Entry Detail
PDB ID:
5LZD
Keywords:
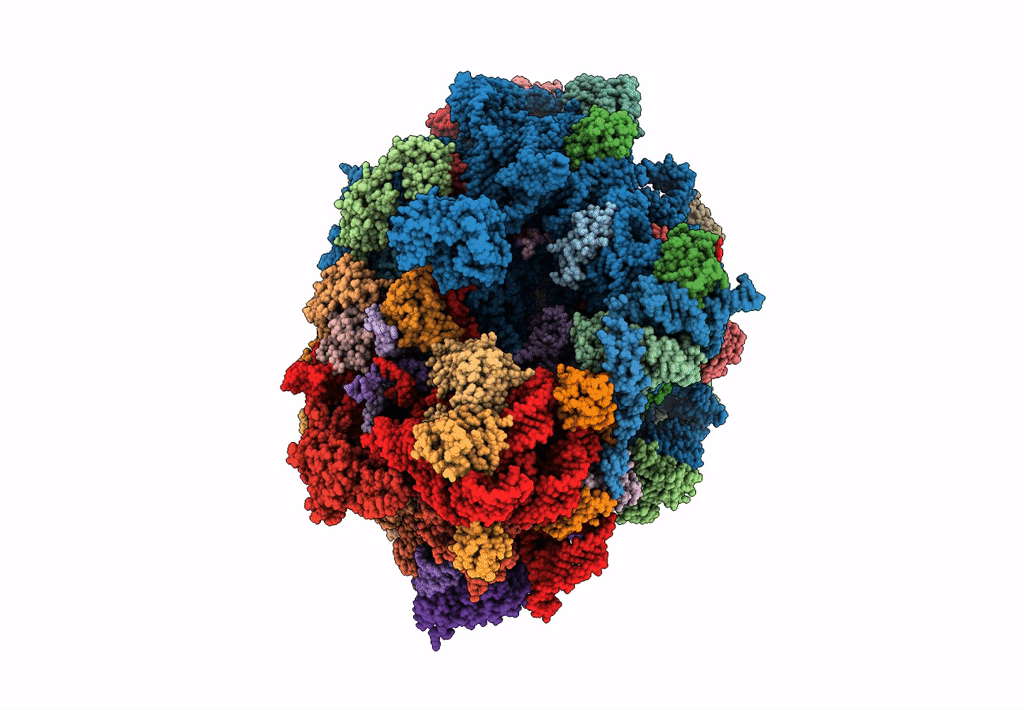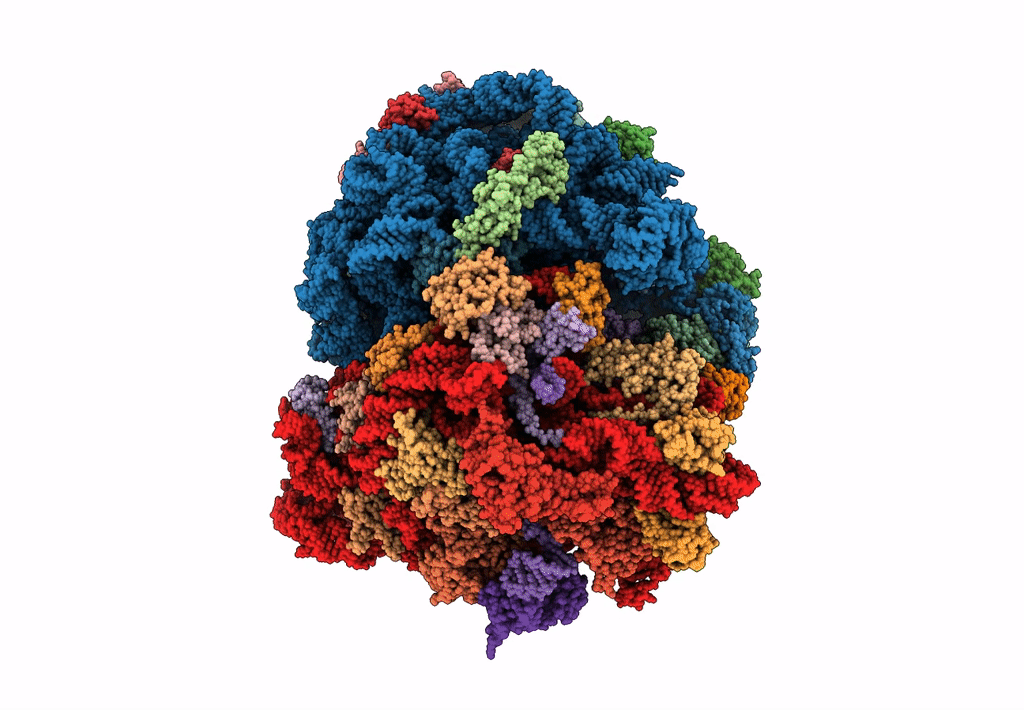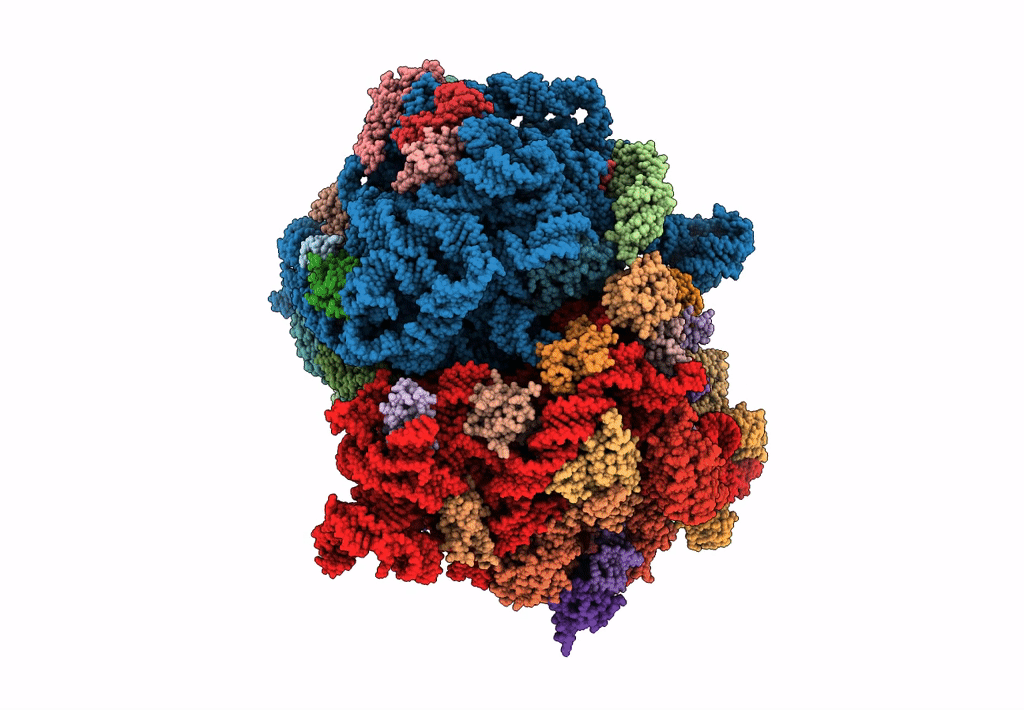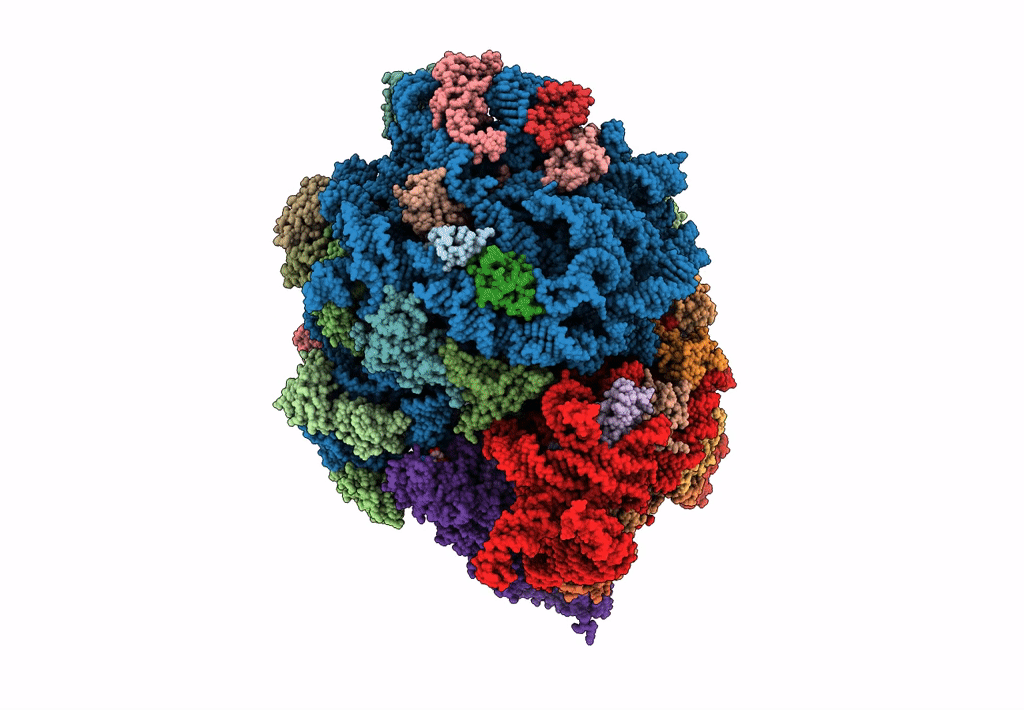
Title:
Structure of SelB-Sec-tRNASec bound to the 70S ribosome in the GTPase activated state (GA)
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Method Details:
Experimental Method:
Resolution:
3.40 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


