Deposition Date
2016-06-14
Release Date
2016-08-31
Last Version Date
2024-11-13
Entry Detail
PDB ID:
5LAS
Keywords:
Title:
HIF prolyl hydroxylase 2 (PHD2-R281C/P317C/R396T) cross-linked to HIF-1alpha NODD-L397C/D412C and N-oxalylglycine (NOG) (complex-3)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
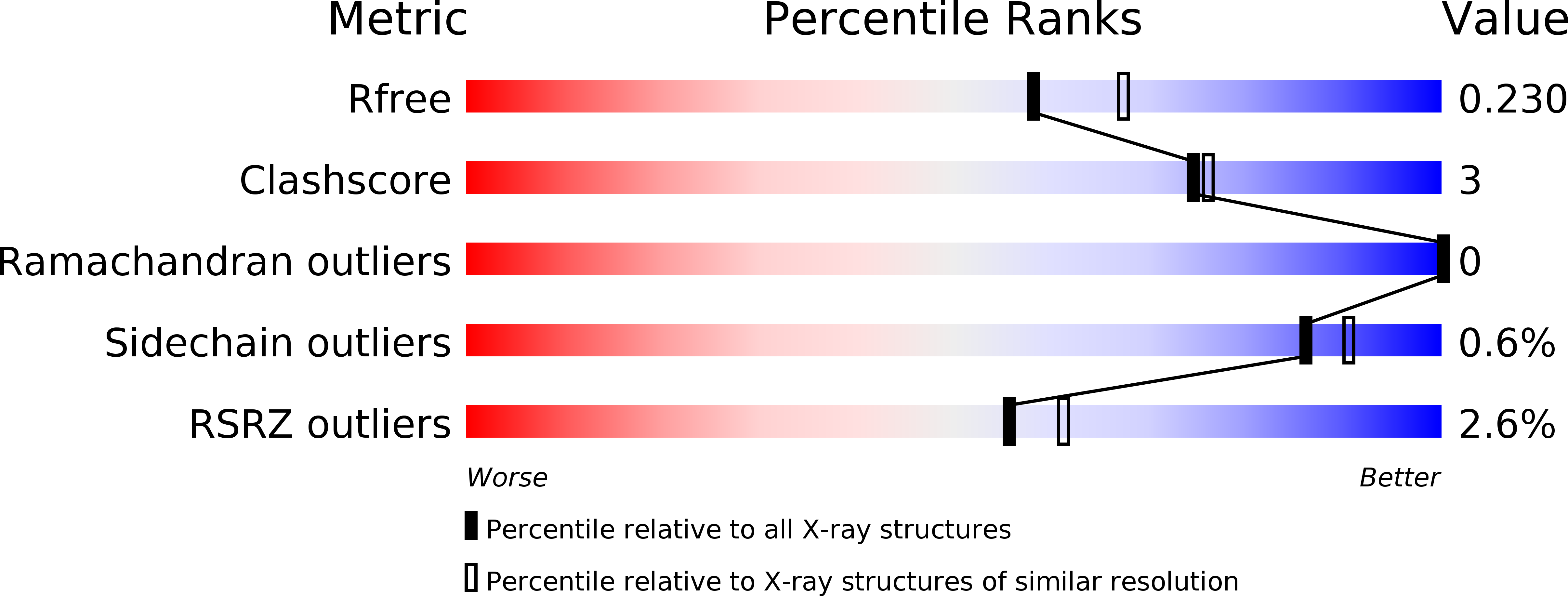
Resolution:
2.10 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 1 21 1