Deposition Date
2016-06-06
Release Date
2016-08-03
Last Version Date
2024-05-08
Entry Detail
PDB ID:
5L83
Keywords:
Title:
Complex of potato ATG8 protein with a peptide from Irish potato famine pathogen effector protein PexRD54
Biological Source:
Source Organism(s):
Solanum tuberosum (Taxon ID: 4113)
Phytophthora infestans (Taxon ID: 4787)
Phytophthora infestans (Taxon ID: 4787)
Expression System(s):
Method Details:
Experimental Method:
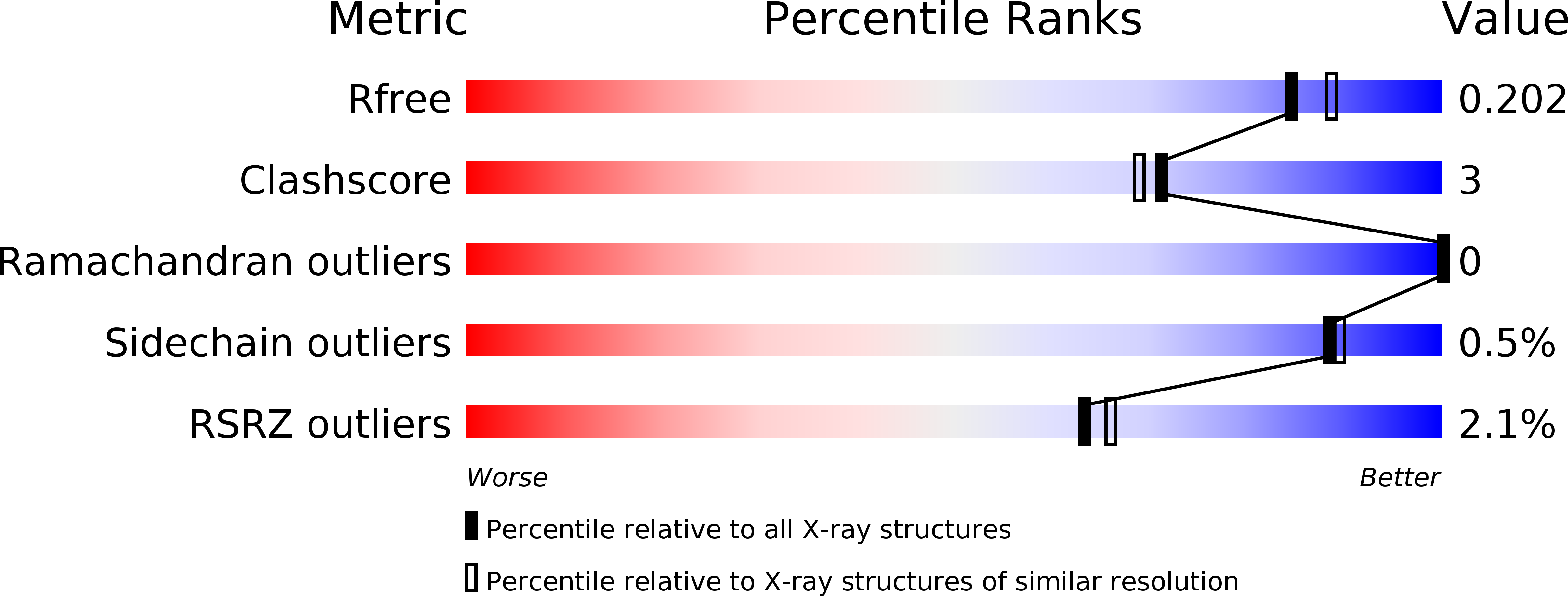
Resolution:
1.90 Å
R-Value Free:
0.19
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
I 41 3 2