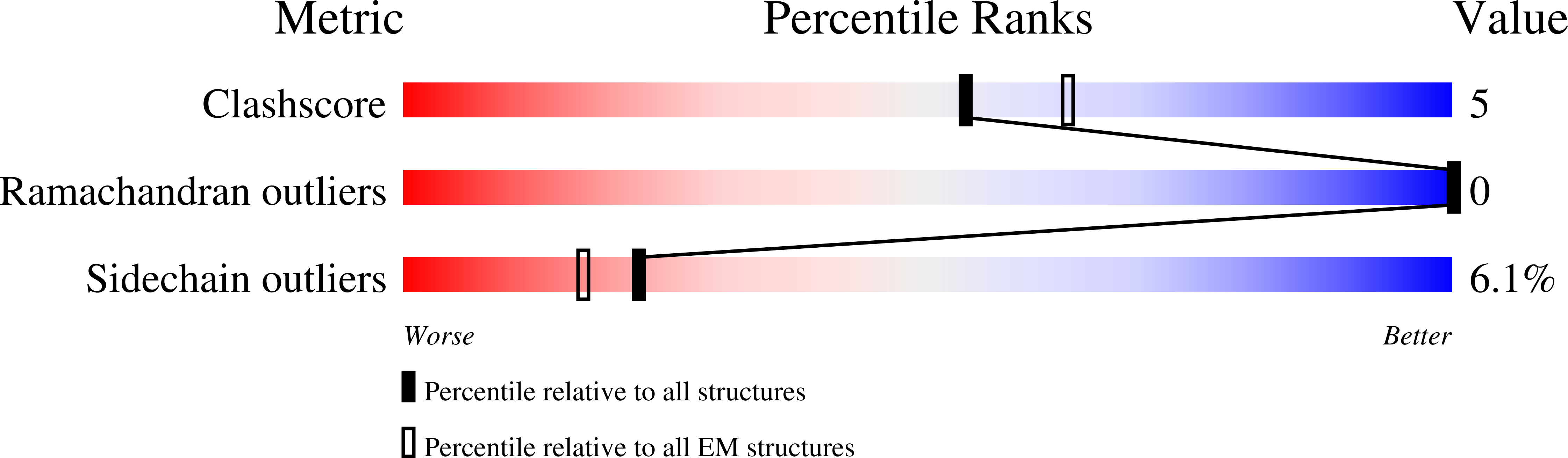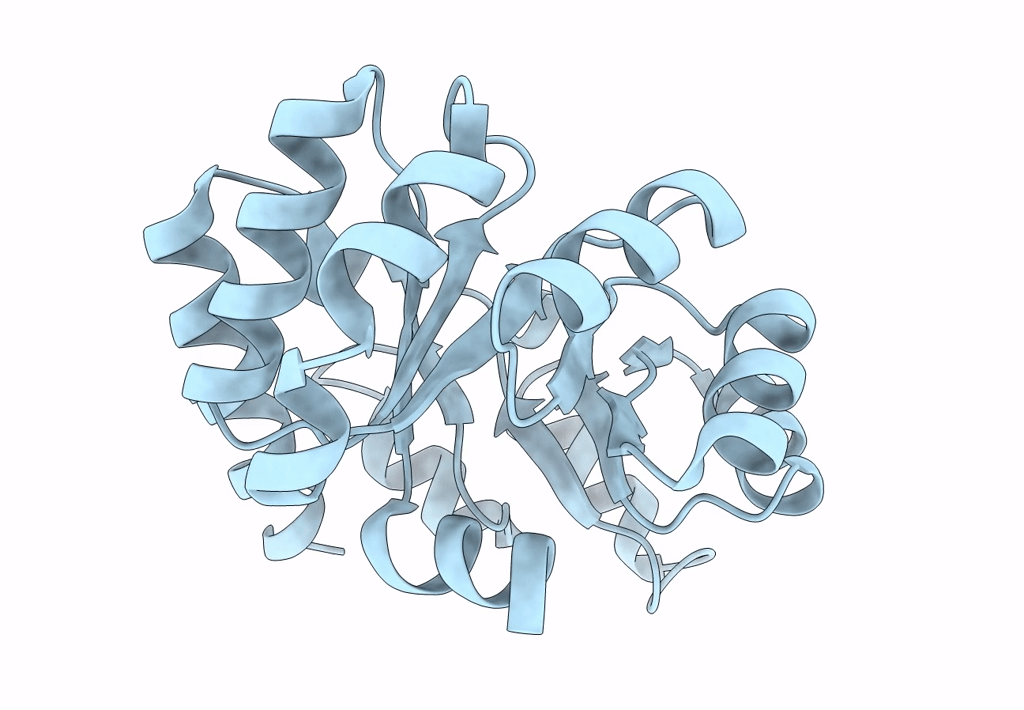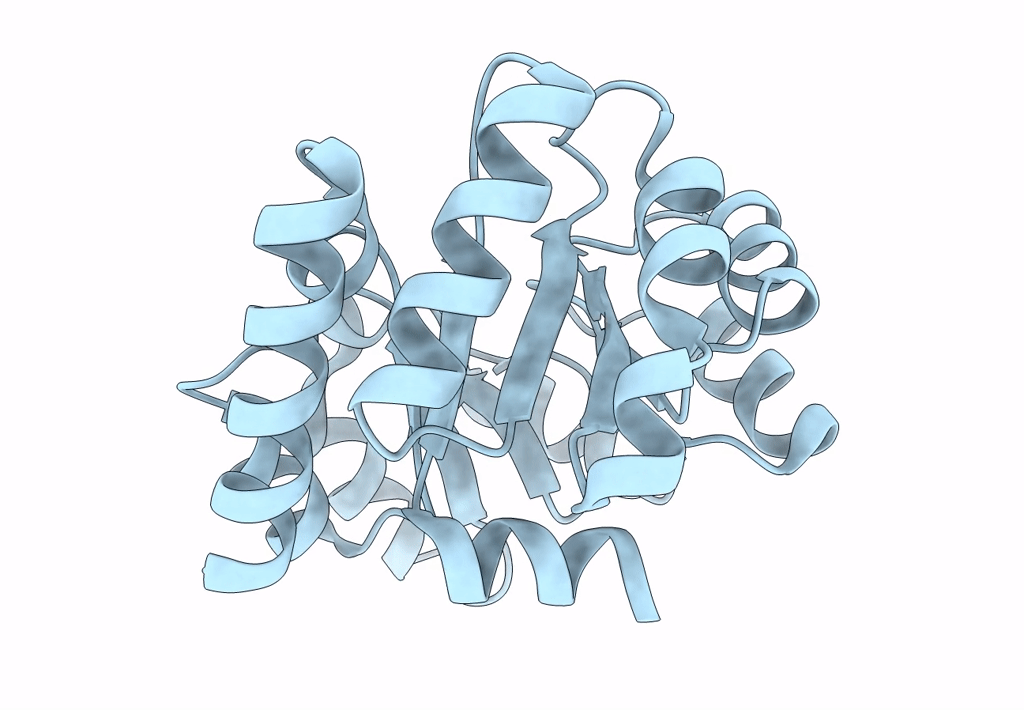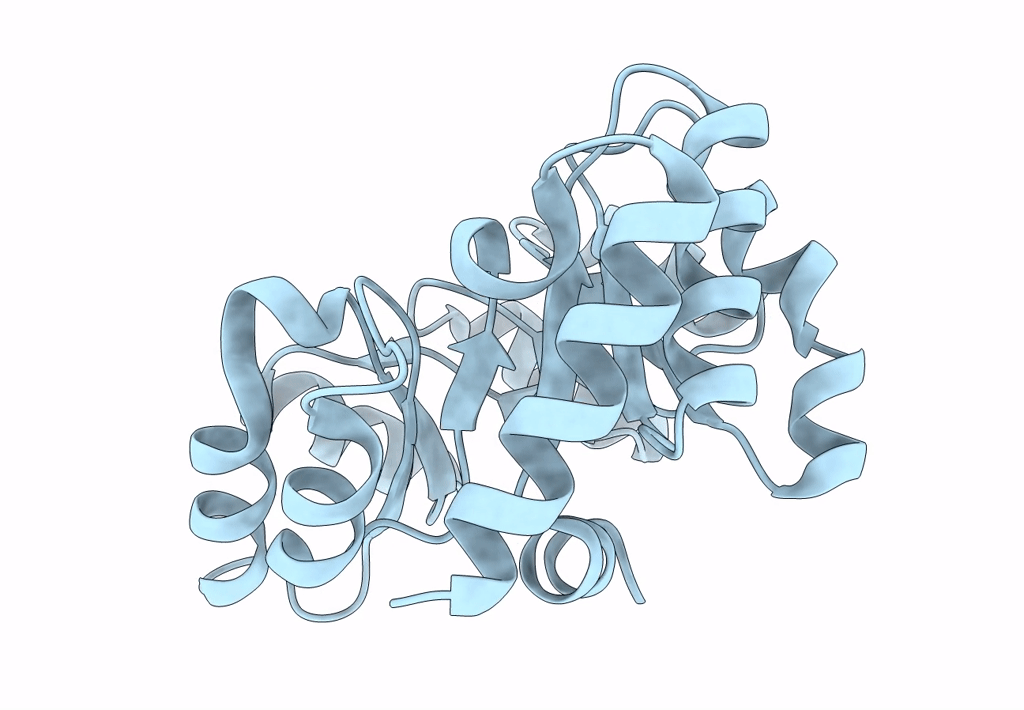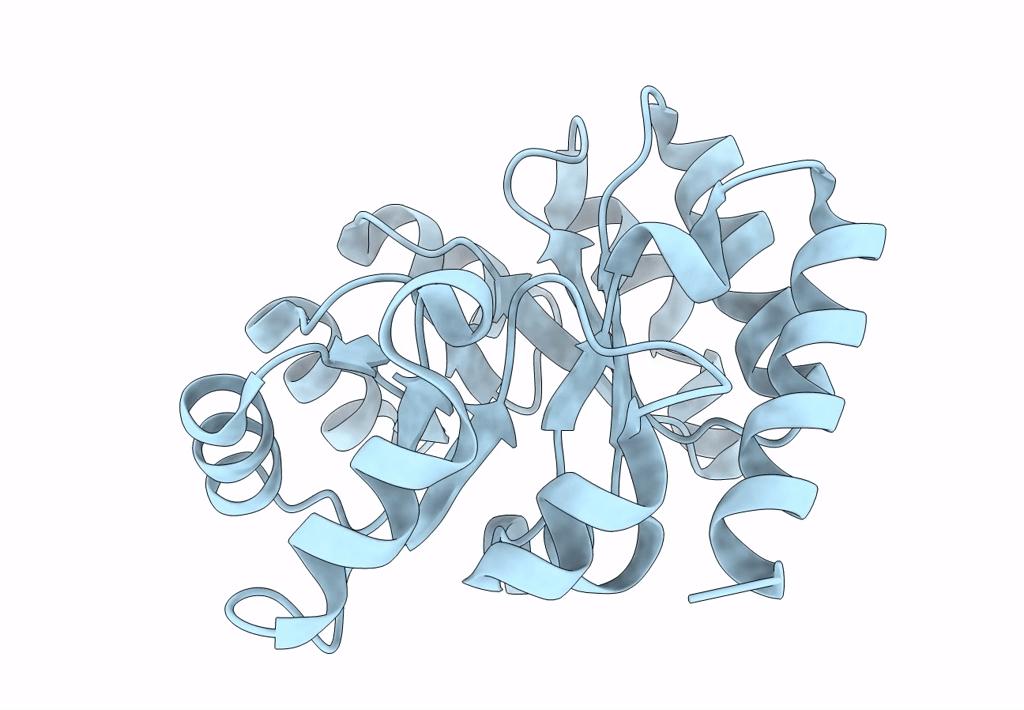
Deposition Date
2016-07-02
Release Date
2016-12-07
Last Version Date
2024-11-13
Entry Detail
PDB ID:
5KP9
Keywords:
Title:
Structure of Nanoparticle Released from Enveloped Protein Nanoparticle
Biological Source:
Source Organism(s):
Thermotoga maritima (Taxon ID: 2336)
Human immunodeficiency virus type 1 group M subtype B (isolate BH10) (Taxon ID: 11678)
Human immunodeficiency virus type 1 group M subtype B (isolate BH10) (Taxon ID: 11678)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
5.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE