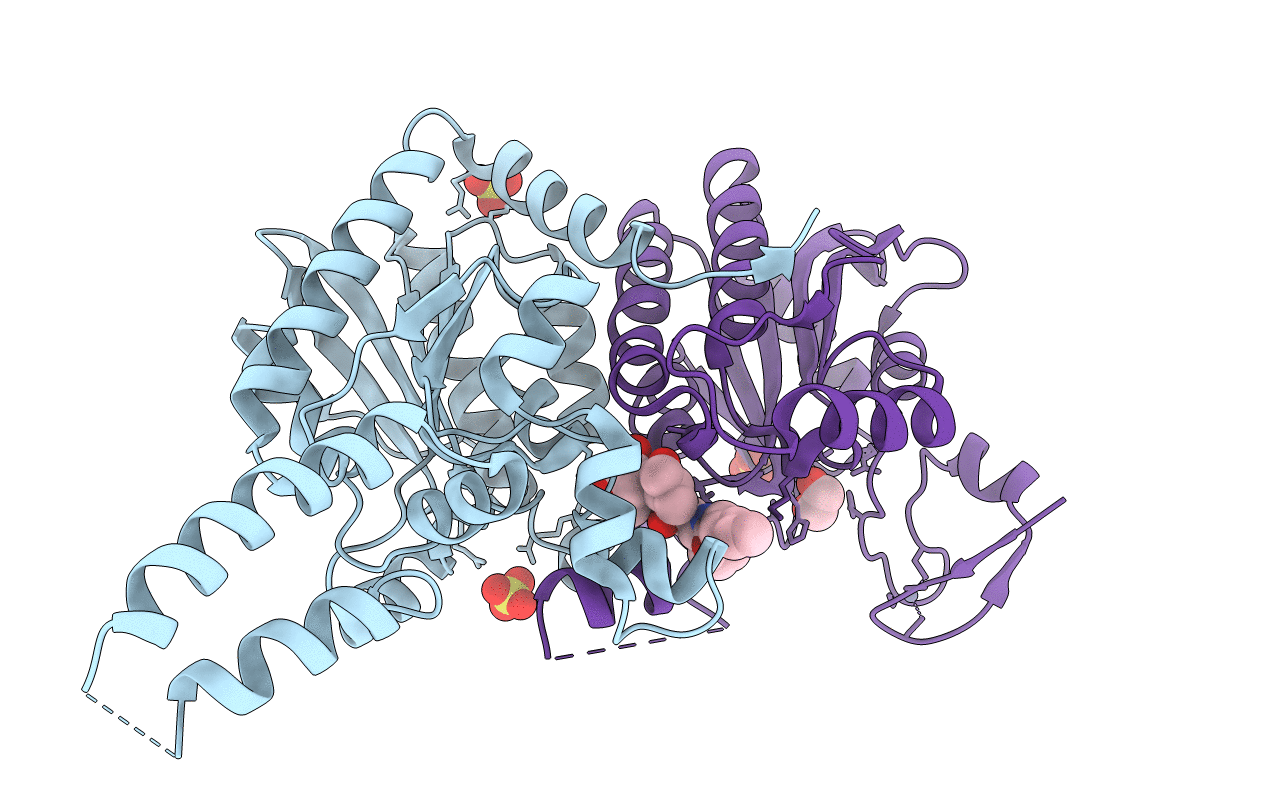
Deposition Date
2016-06-08
Release Date
2016-08-10
Last Version Date
2023-09-27
Entry Detail
PDB ID:
5KDR
Keywords:
Title:
The crystal structure of carboxyltransferase from Staphylococcus Aureus bound to the antimicrobial agent moiramide B.
Biological Source:
Source Organism(s):
Staphylococcus aureus (strain USA300) (Taxon ID: 367830)
Staphylococcus aureus (strain bovine RF122 / ET3-1) (Taxon ID: 273036)
Staphylococcus aureus (strain bovine RF122 / ET3-1) (Taxon ID: 273036)
Expression System(s):
Method Details:
Experimental Method:
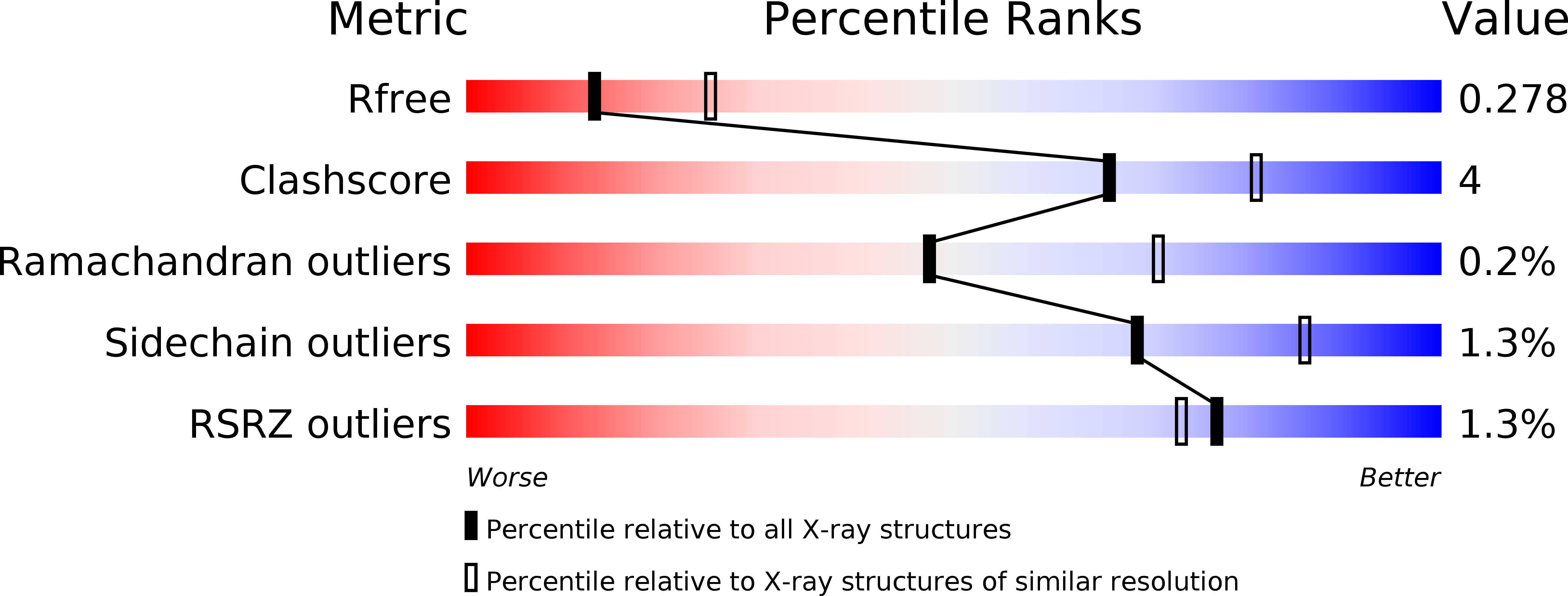
Resolution:
2.60 Å
R-Value Free:
0.27
R-Value Work:
0.22
R-Value Observed:
0.23
Space Group:
C 1 2 1