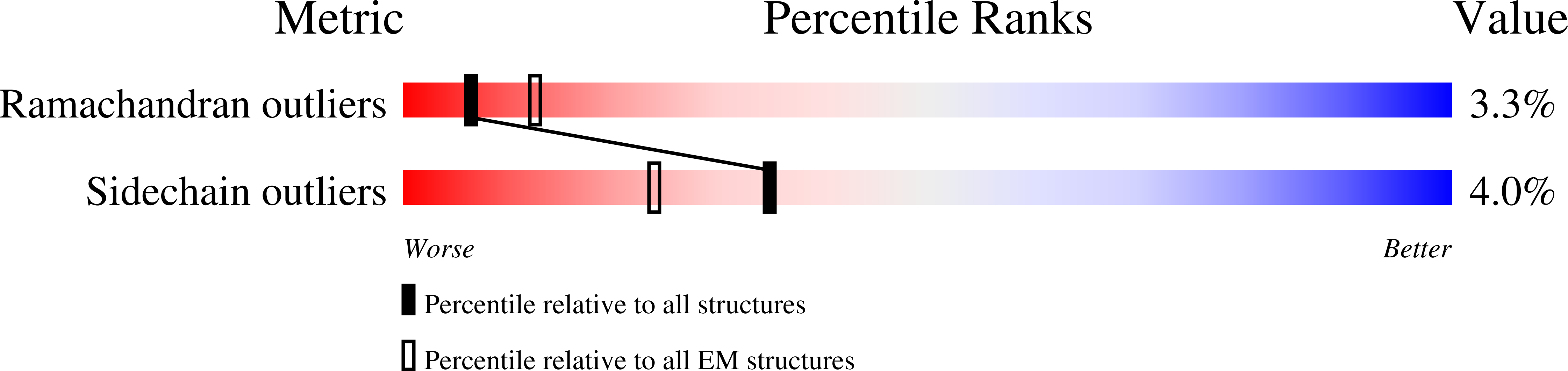Deposition Date
2016-05-18
Release Date
2016-07-13
Last Version Date
2024-05-08
Entry Detail
PDB ID:
5K1H
Keywords:
Title:
eIF3b relocated to the intersubunit face to interact with eIF1 and below the eIF2 ternary-complex. from the structure of a partial yeast 48S preinitiation complex in closed conformation.
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Oryctolagus cuniculus (Taxon ID: 9986)
Oryctolagus cuniculus (Taxon ID: 9986)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE