Deposition Date
2016-05-13
Release Date
2017-01-11
Last Version Date
2024-10-30
Entry Detail
PDB ID:
5JYC
Keywords:
Title:
Crystal structure of the E153Q mutant of the CFTR inhibitory factor Cif containing the adducted 14,15-EET hydrolysis intermediate
Biological Source:
Source Organism(s):
Pseudomonas aeruginosa (strain UCBPP-PA14) (Taxon ID: 208963)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.00 Å
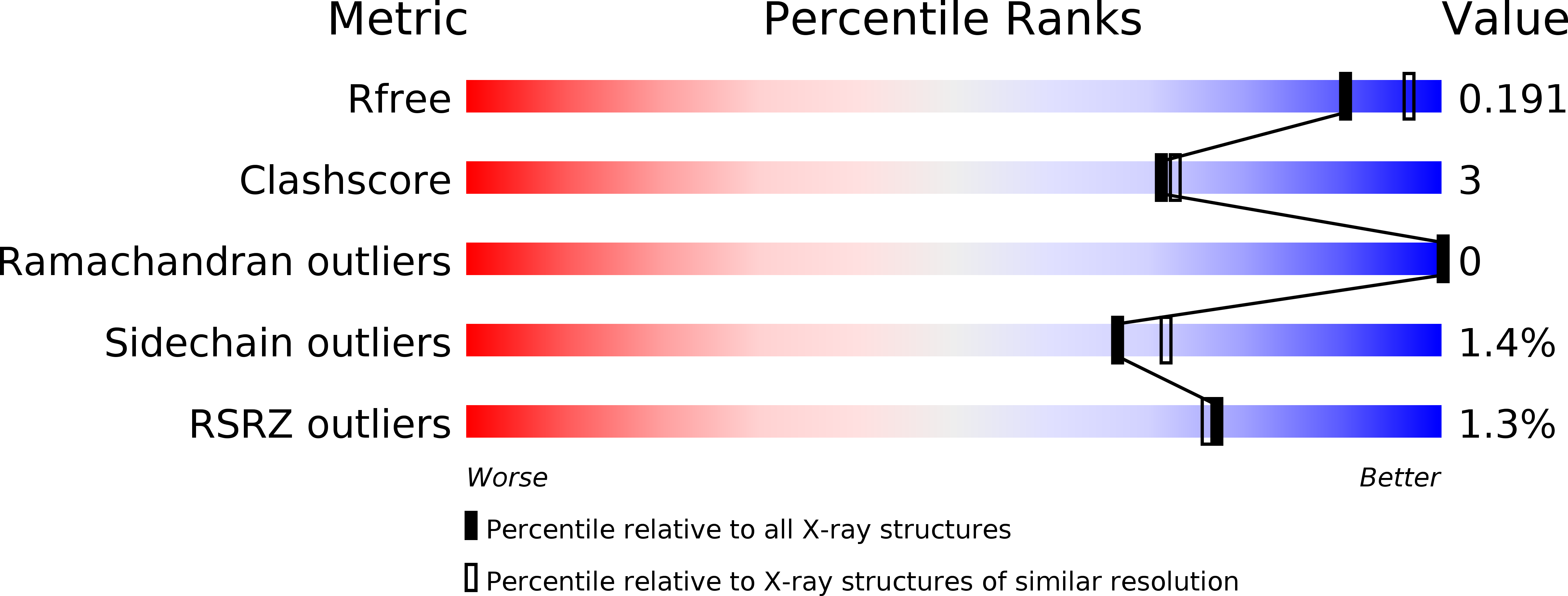
R-Value Free:
0.19
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
C 1 2 1