Deposition Date
2016-05-05
Release Date
2016-09-28
Last Version Date
2023-09-27
Entry Detail
PDB ID:
5JQU
Keywords:
Title:
Crystal structure of Cytochrome P450 BM3 heme domain G265F/T269V/L272W/L322I/F405M/A406S (WIVS-FM) variant with iron(III) deuteroporphyrin IX bound
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
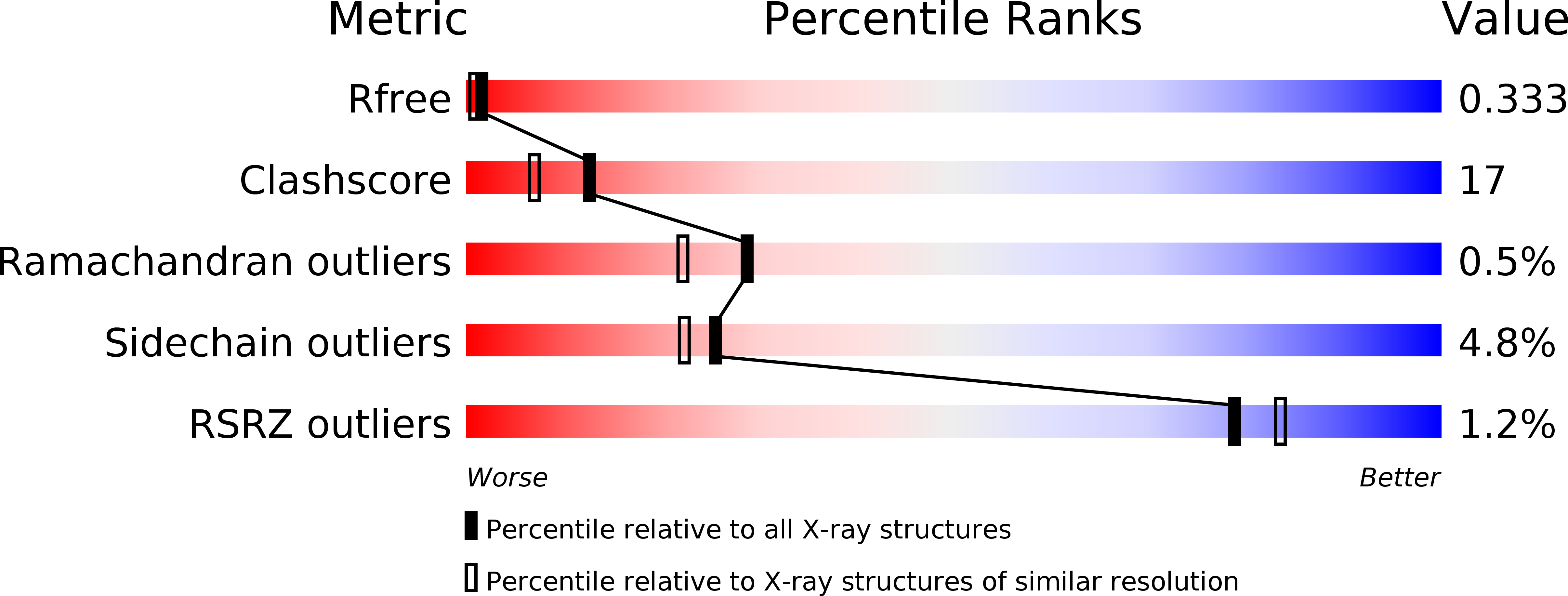
Resolution:
2.16 Å
R-Value Free:
0.33
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
P 21 21 21