Deposition Date
2016-04-27
Release Date
2016-08-03
Last Version Date
2024-10-23
Entry Detail
PDB ID:
5JLG
Keywords:
Title:
The X-ray structure of the adduct formed in the reaction between bovine pancreatic ribonuclease and compound I, a piano-stool organometallic Ru(II) arene compound containing an O,S-chelating ligand
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
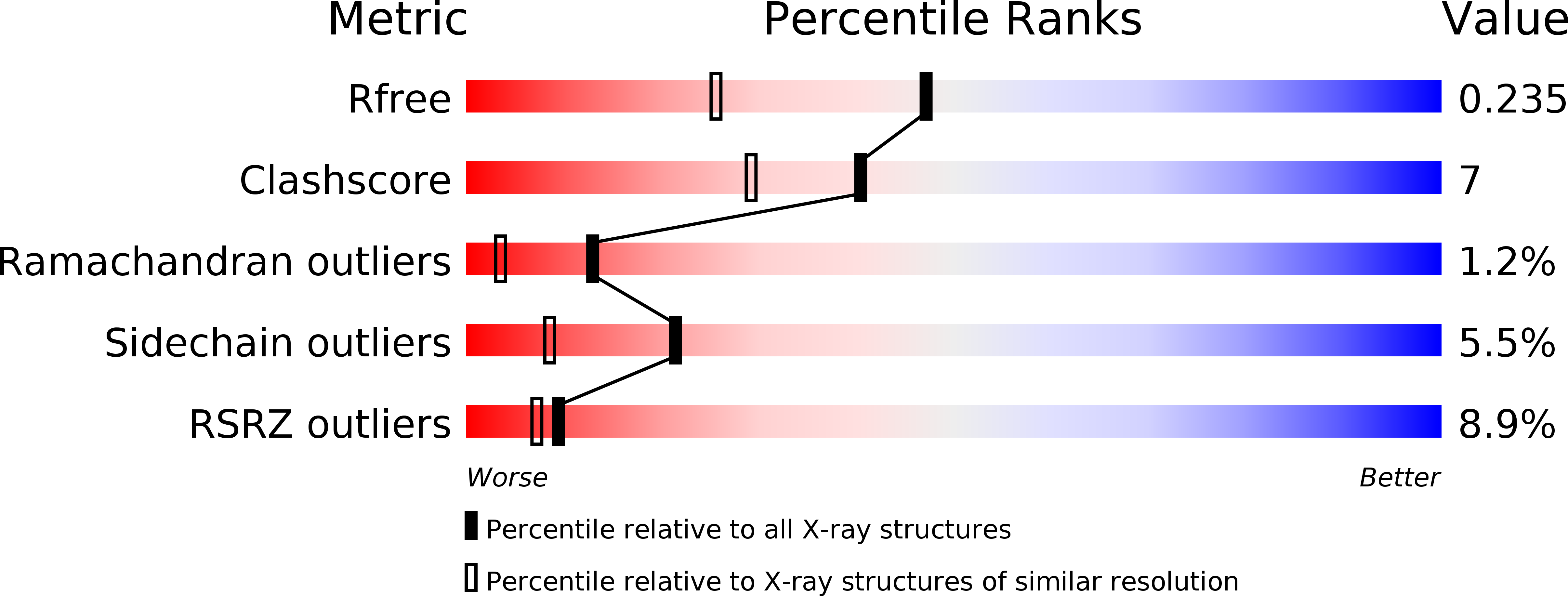
Resolution:
1.79 Å
R-Value Free:
0.23
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1