Deposition Date
2016-04-20
Release Date
2017-07-05
Last Version Date
2024-03-06
Entry Detail
PDB ID:
5JGC
Keywords:
Title:
Crystal structure of the rice Topless related protein 2 (TPR2) N-terminal topless domain (1-209) L111A, L130A, L179A and I195A mutant
Biological Source:
Source Organism(s):
Oryza sativa (Taxon ID: 4530)
Expression System(s):
Method Details:
Experimental Method:
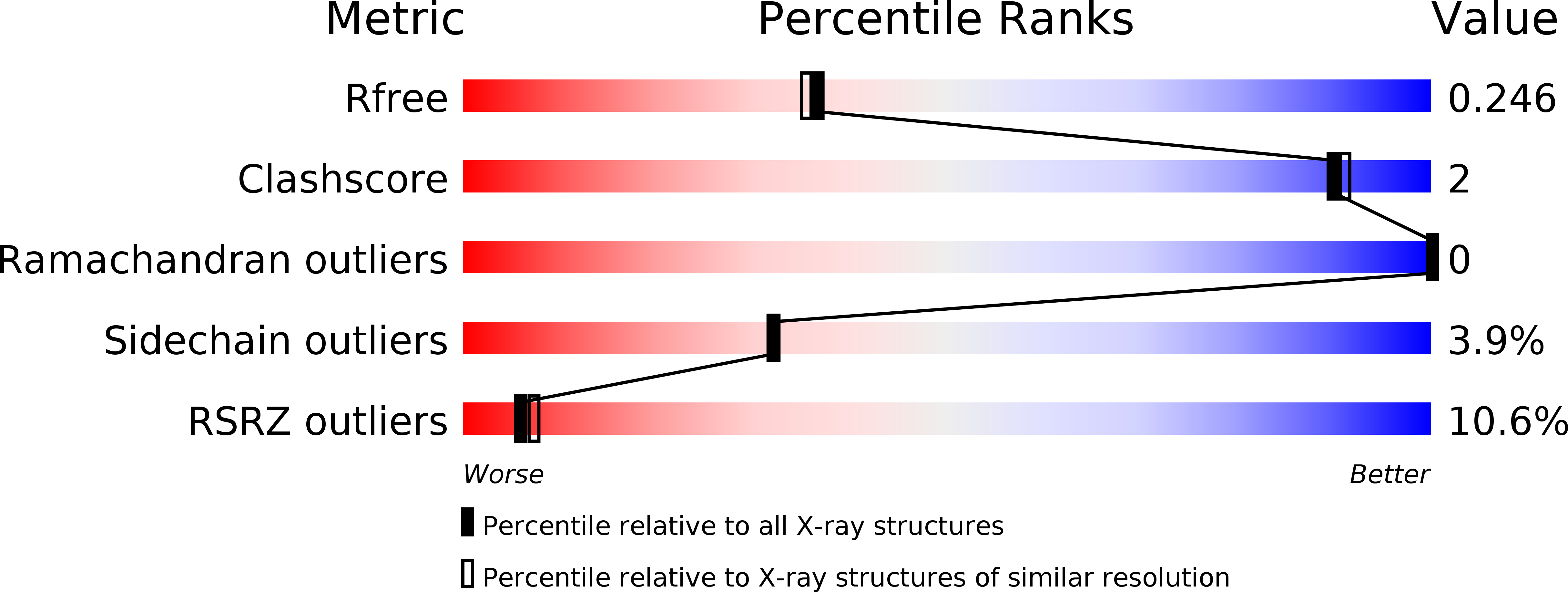
Resolution:
2.08 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.21
Space Group:
P 42 21 2