Deposition Date
2016-04-17
Release Date
2016-05-11
Last Version Date
2023-09-27
Entry Detail
PDB ID:
5JDQ
Keywords:
Title:
Structural mechanisms of extracellular ion exchange and induced binding-site occlusion in the sodium-calcium exchanger NCX_Mj soaked with 100 mM Na+ and 10mM Sr2+
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
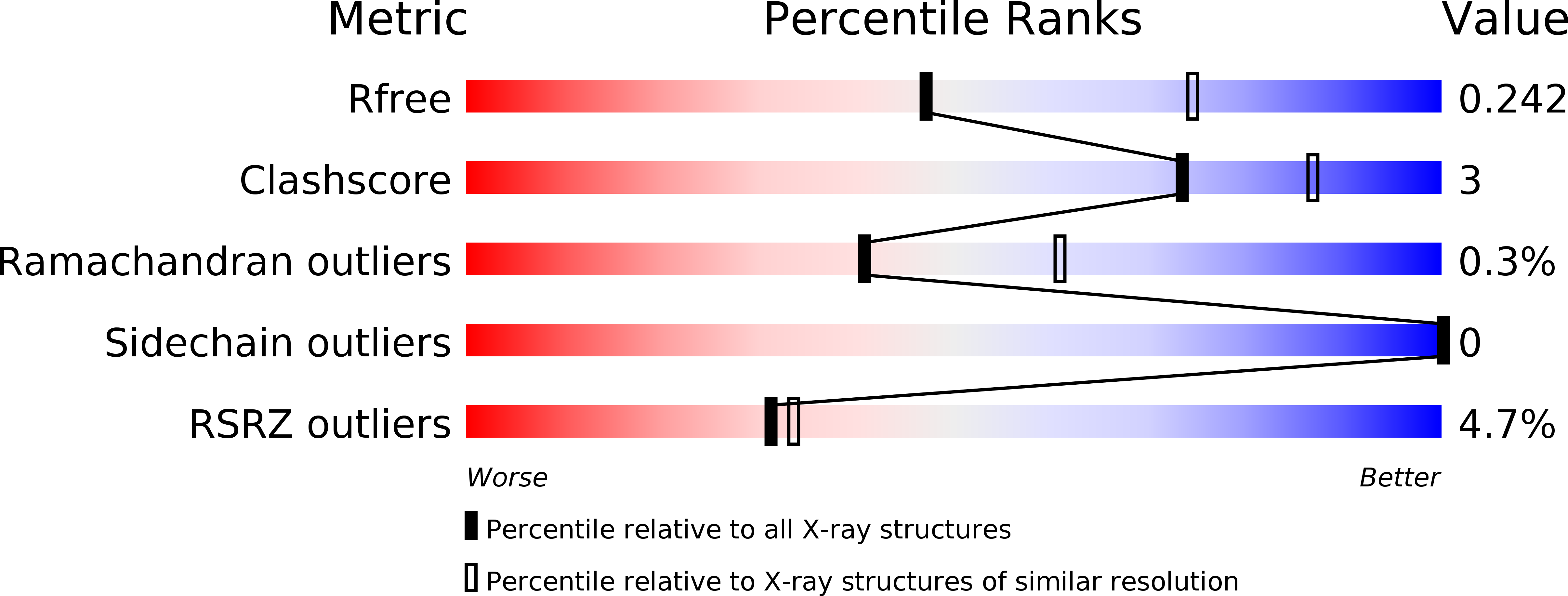
Resolution:
2.50 Å
R-Value Free:
0.24
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 21 21 21