Deposition Date
2016-04-14
Release Date
2016-06-01
Last Version Date
2024-01-10
Entry Detail
PDB ID:
5JC7
Keywords:
Title:
Crystal structure of chicken MDA5 with 5'p 24-mer dsRNA and ADP-Mg2+ at 2.75 A resolution.
Biological Source:
Source Organism(s):
Gallus gallus (Taxon ID: 9031)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.75 Å
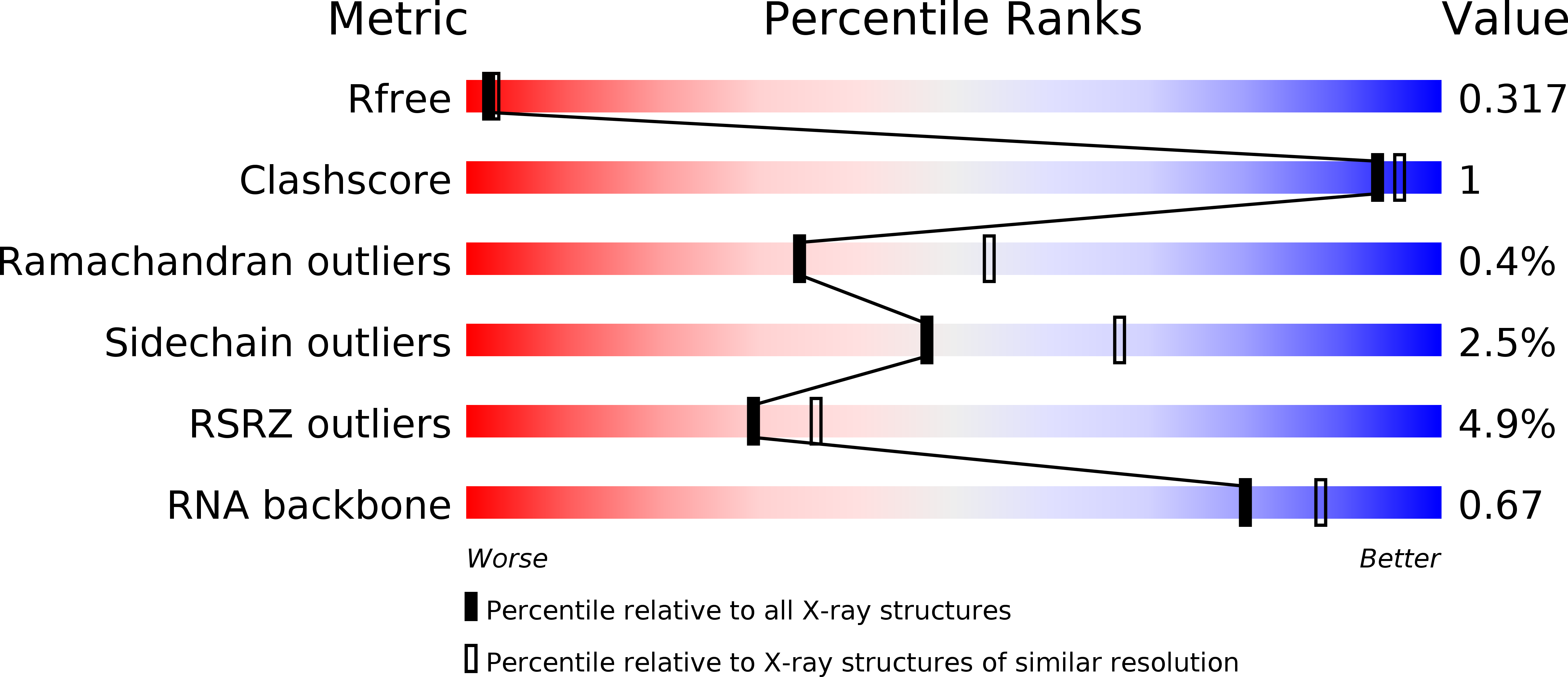
R-Value Free:
0.32
R-Value Work:
0.29
R-Value Observed:
0.29
Space Group:
P 21 21 21