Deposition Date
2016-03-31
Release Date
2017-04-12
Last Version Date
2024-10-16
Entry Detail
PDB ID:
5J3N
Keywords:
Title:
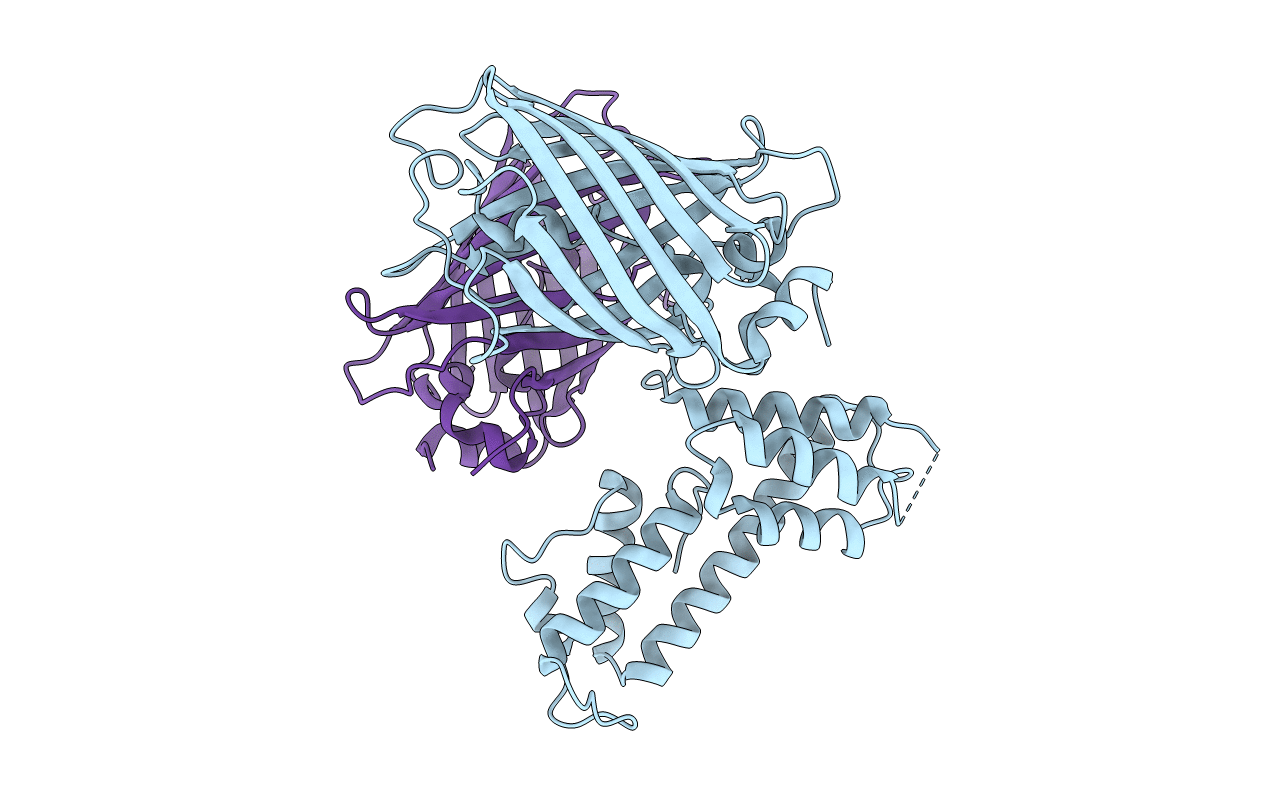
C-terminal domain of EcoR124I HsdR subunit fused with the pH-sensitive GFP variant ratiometric pHluorin
Biological Source:
Source Organism(s):
Aequorea victoria (Taxon ID: 6100)
Escherichia coli (Taxon ID: 562)
Escherichia coli (Taxon ID: 562)
Expression System(s):
Method Details:
Experimental Method:
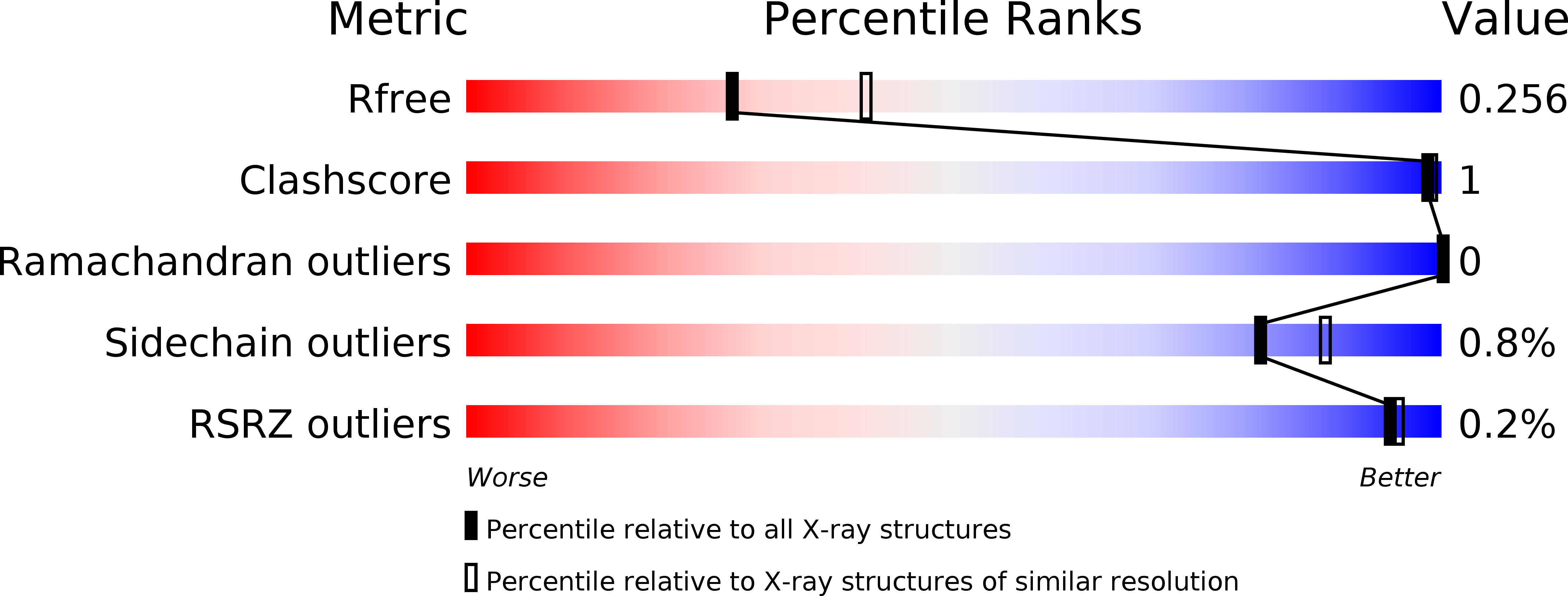
Resolution:
2.45 Å
R-Value Free:
0.25
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 2 2 21