Deposition Date
2016-03-29
Release Date
2016-08-03
Last Version Date
2025-07-02
Entry Detail
PDB ID:
5J2P
Keywords:
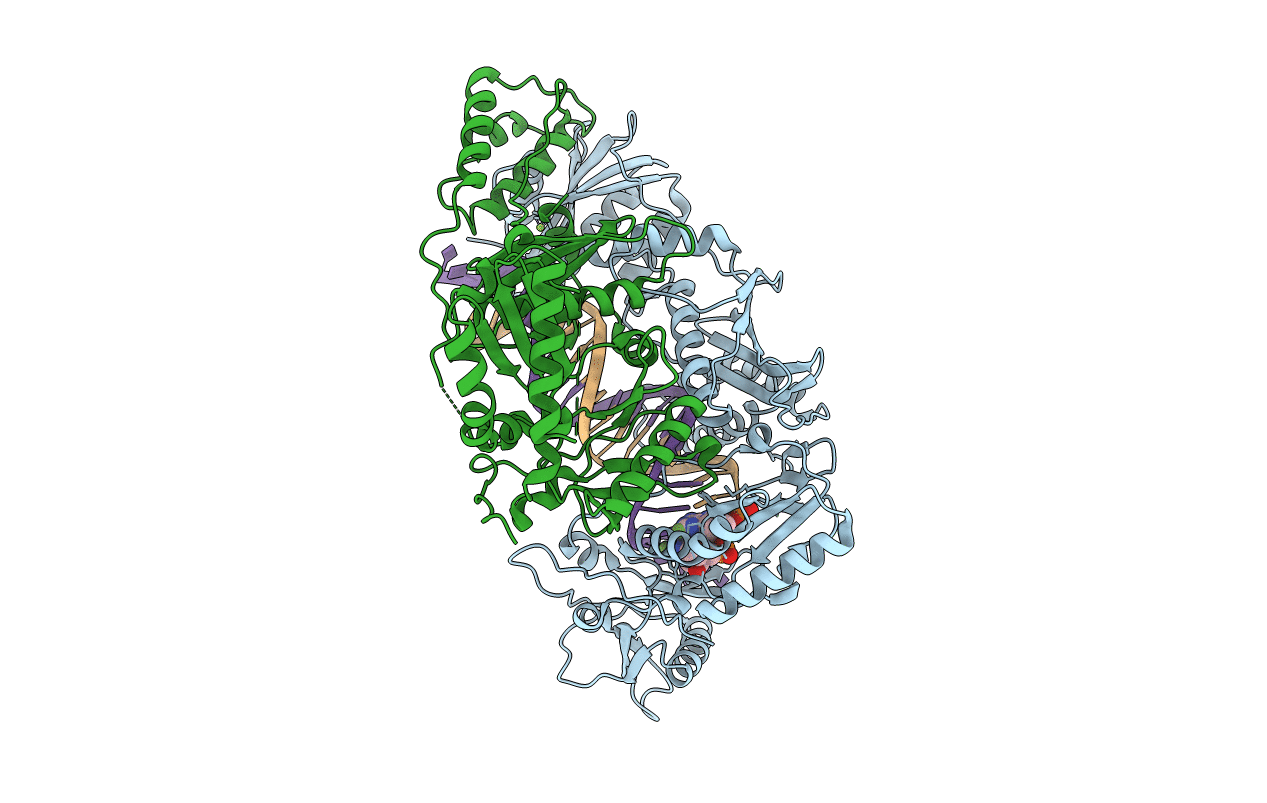
Title:
HIV-1 reverse transcriptase in complex with DNA that has incorporated EFdA-MP at the P-(post-translocation) site and a second EFdA-MP at the N-(pre-translocation) site
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.53 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 2 2 21


