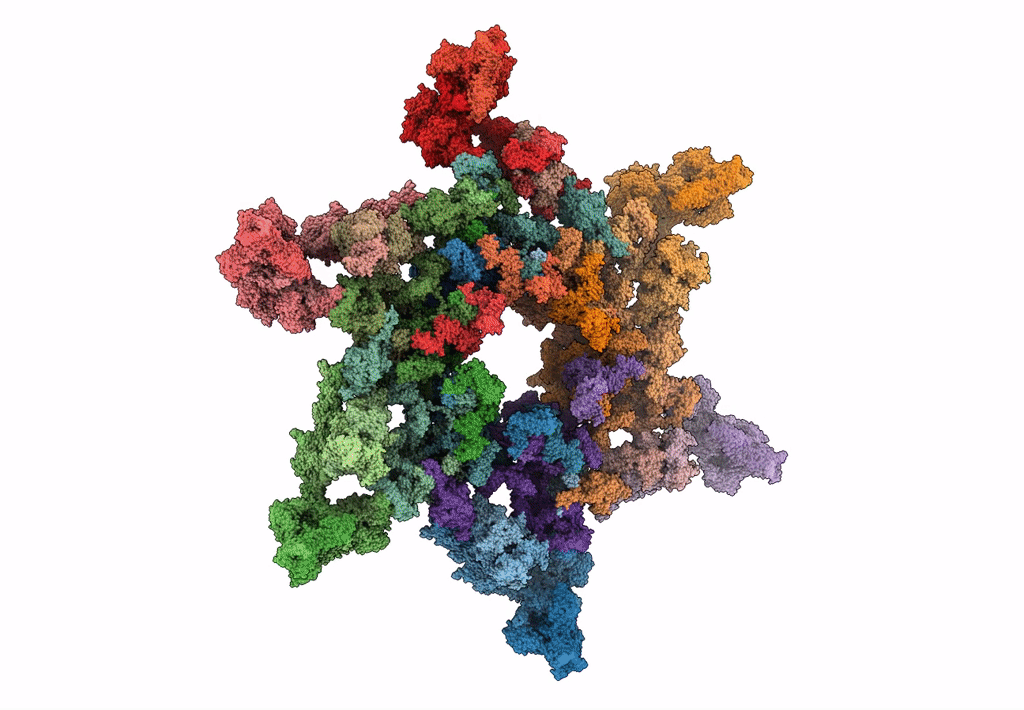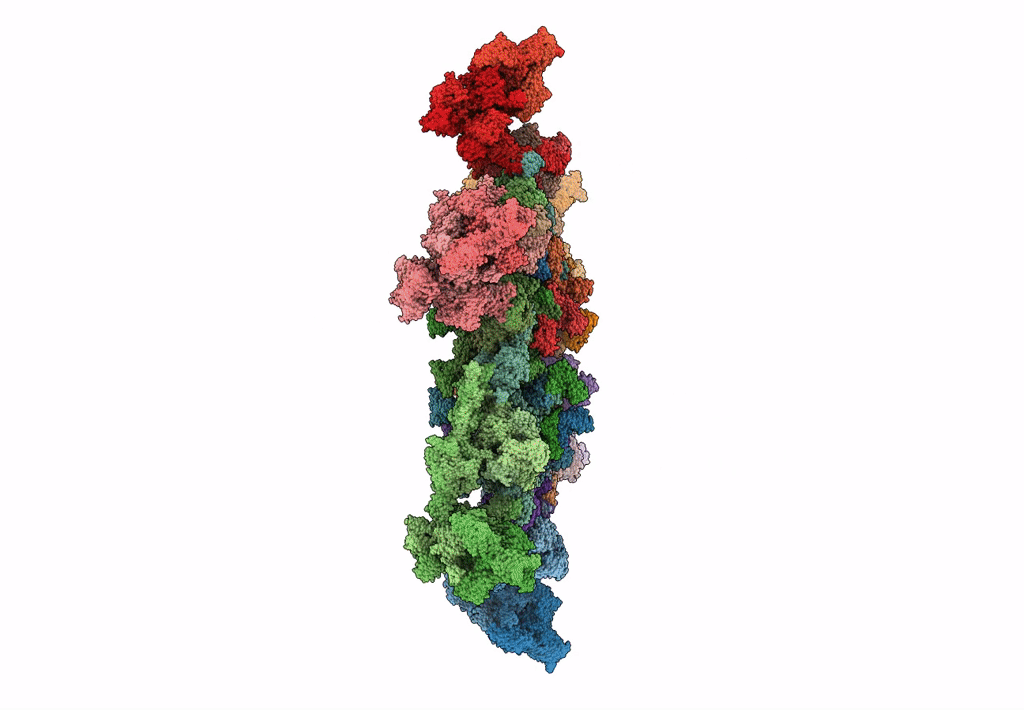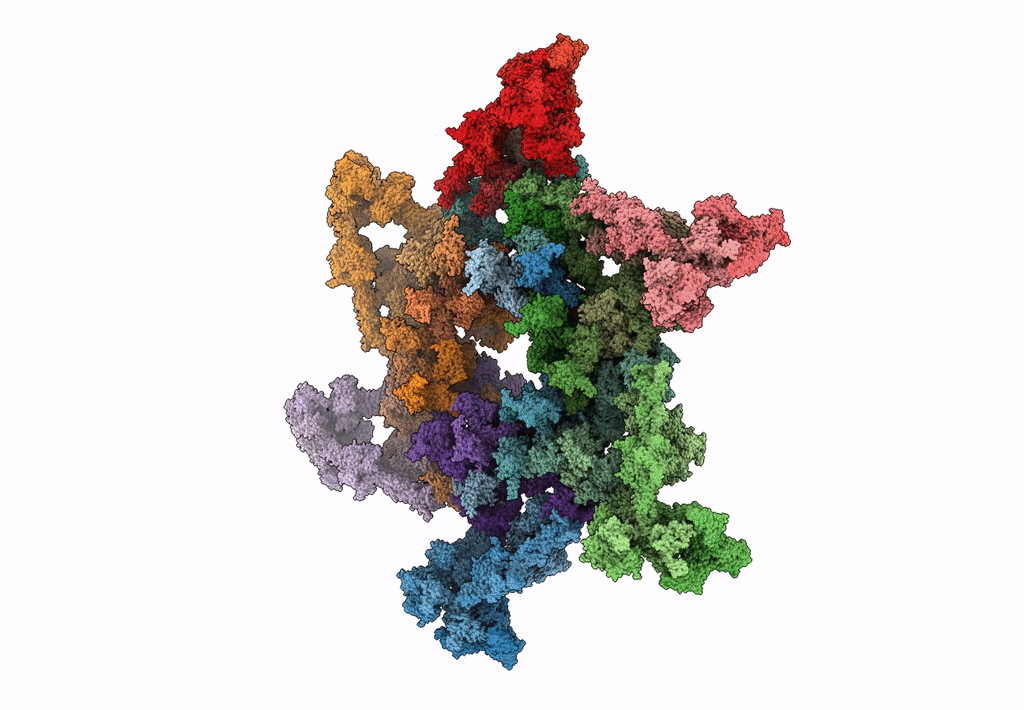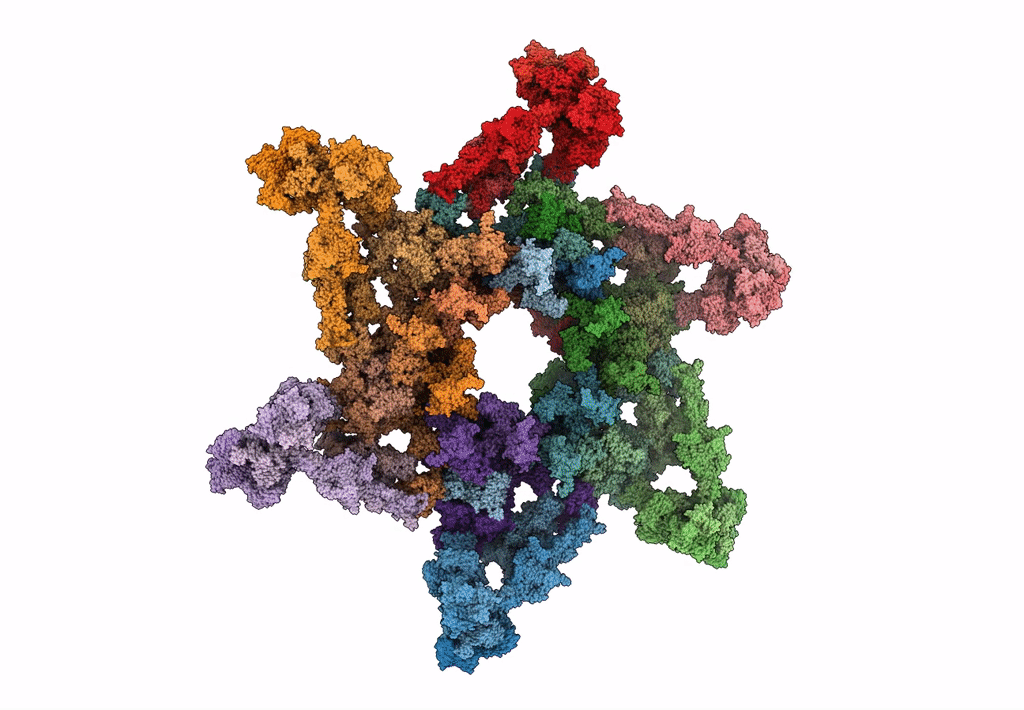
Deposition Date
2016-03-19
Release Date
2016-05-18
Last Version Date
2024-11-20
Entry Detail
PDB ID:
5IV7
Keywords:
Title:
Cryo-electron microscopy structure of the star-shaped, hubless post-attachment T4 baseplate
Biological Source:
Source Organism(s):
Enterobacteria phage T4 (Taxon ID: 10665)
Method Details:
Experimental Method:
Resolution:
6.77 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE