Deposition Date
2016-02-08
Release Date
2016-06-01
Last Version Date
2024-05-22
Entry Detail
PDB ID:
5I2G
Keywords:
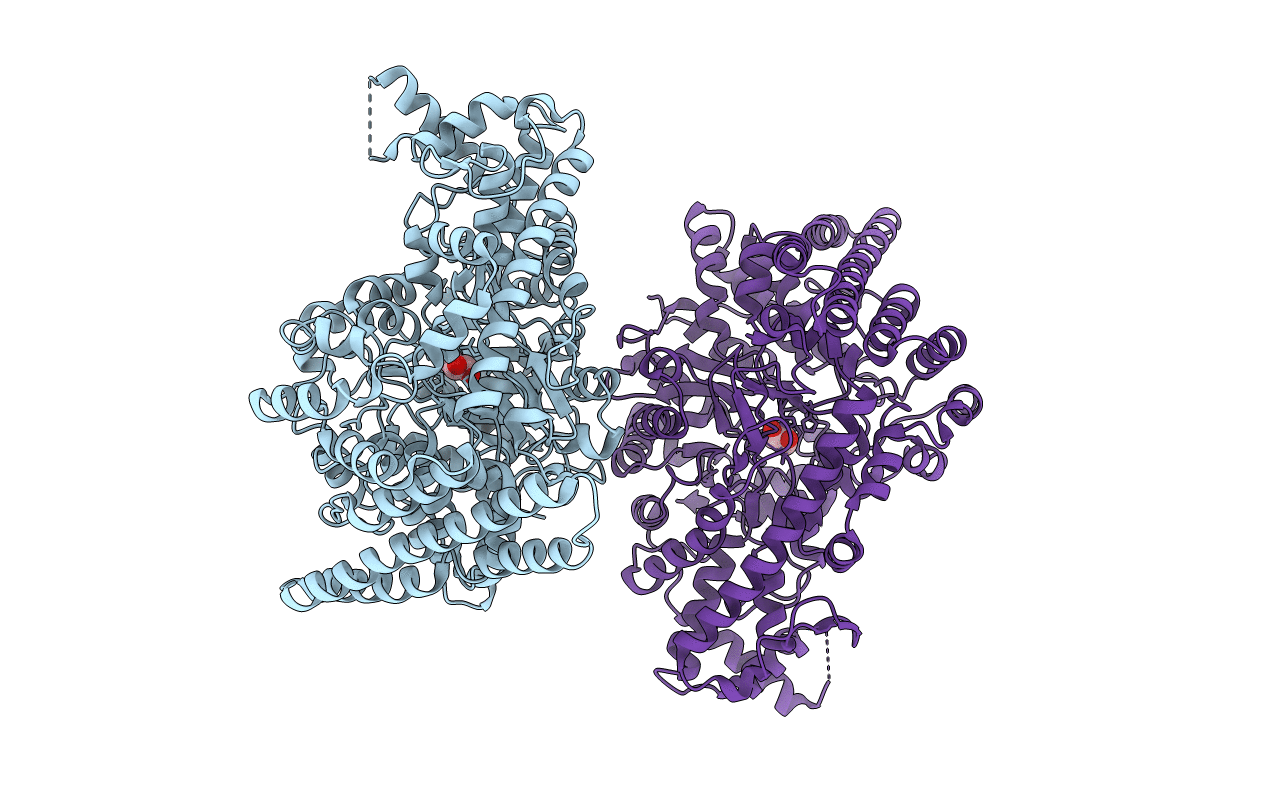
Title:
1,2-propanediol Dehydration in Roseburia inulinivorans; Structural Basis for Substrate and Enantiomer Selectivity
Biological Source:
Source Organism(s):
Roseburia inulinivorans (Taxon ID: 360807)
Expression System(s):
Method Details:
Experimental Method:
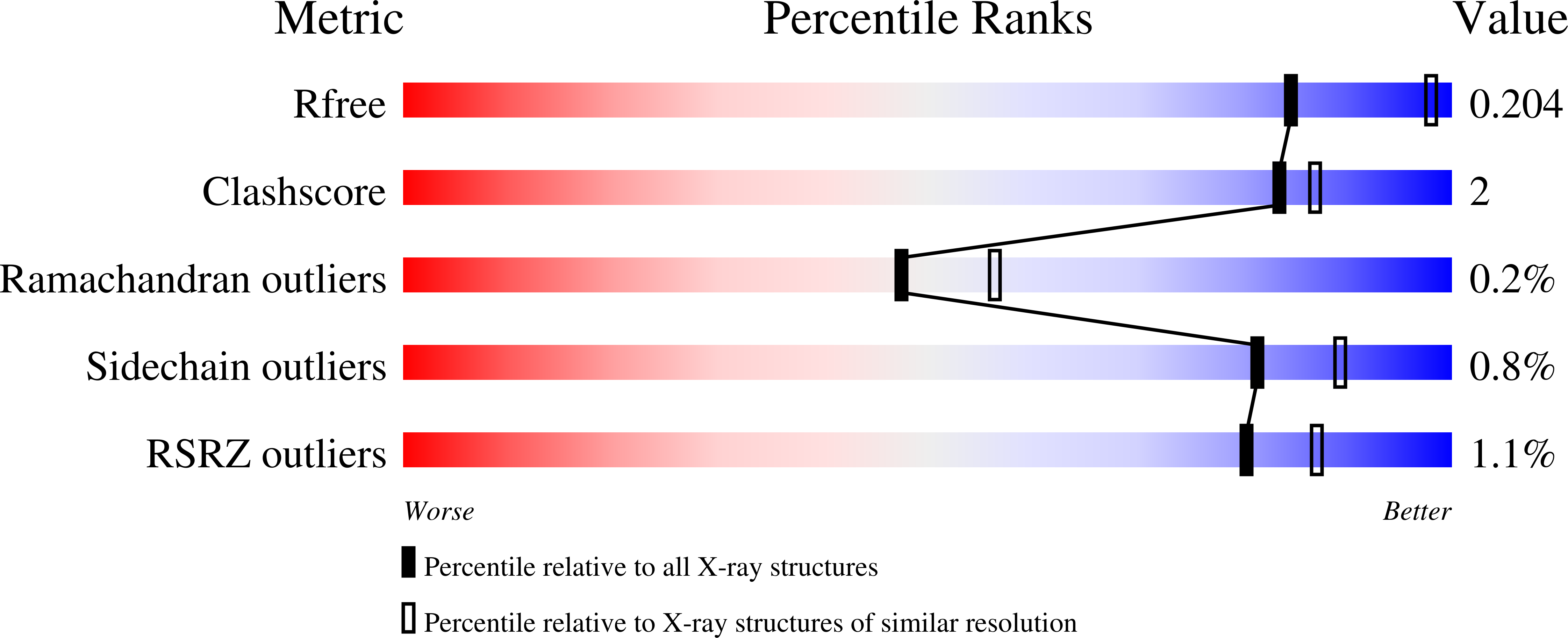
Resolution:
2.35 Å
R-Value Free:
0.20
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
C 1 2 1