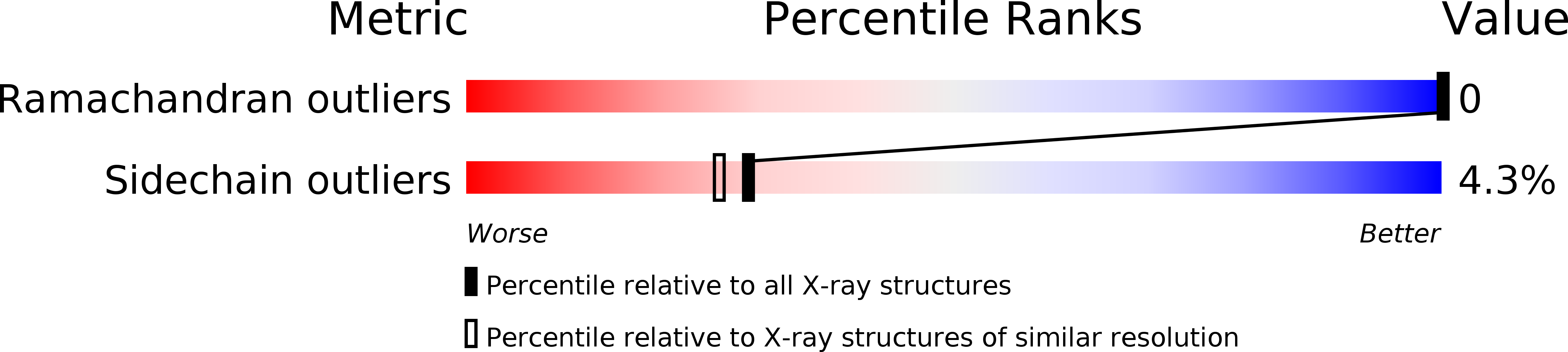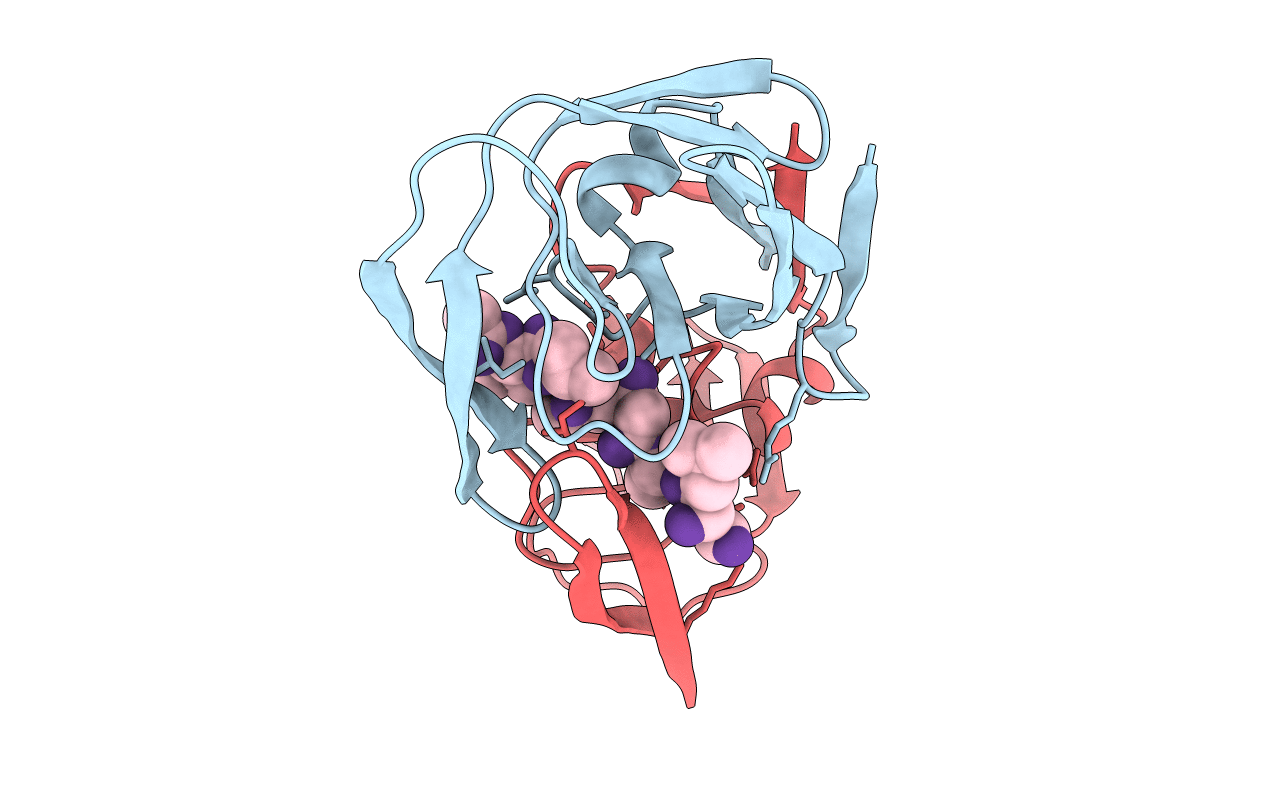
Deposition Date
1990-04-30
Release Date
1991-10-15
Last Version Date
2024-11-20
Entry Detail
PDB ID:
5HVP
Keywords:
Title:
CRYSTALLOGRAPHIC ANALYSIS OF A COMPLEX BETWEEN HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 PROTEASE AND ACETYL-PEPSTATIN AT 2.0-ANGSTROMS RESOLUTION
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Streptomyces (Taxon ID: 1883)
Streptomyces (Taxon ID: 1883)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Observed:
0.17
Space Group:
P 21 21 2