Abstact
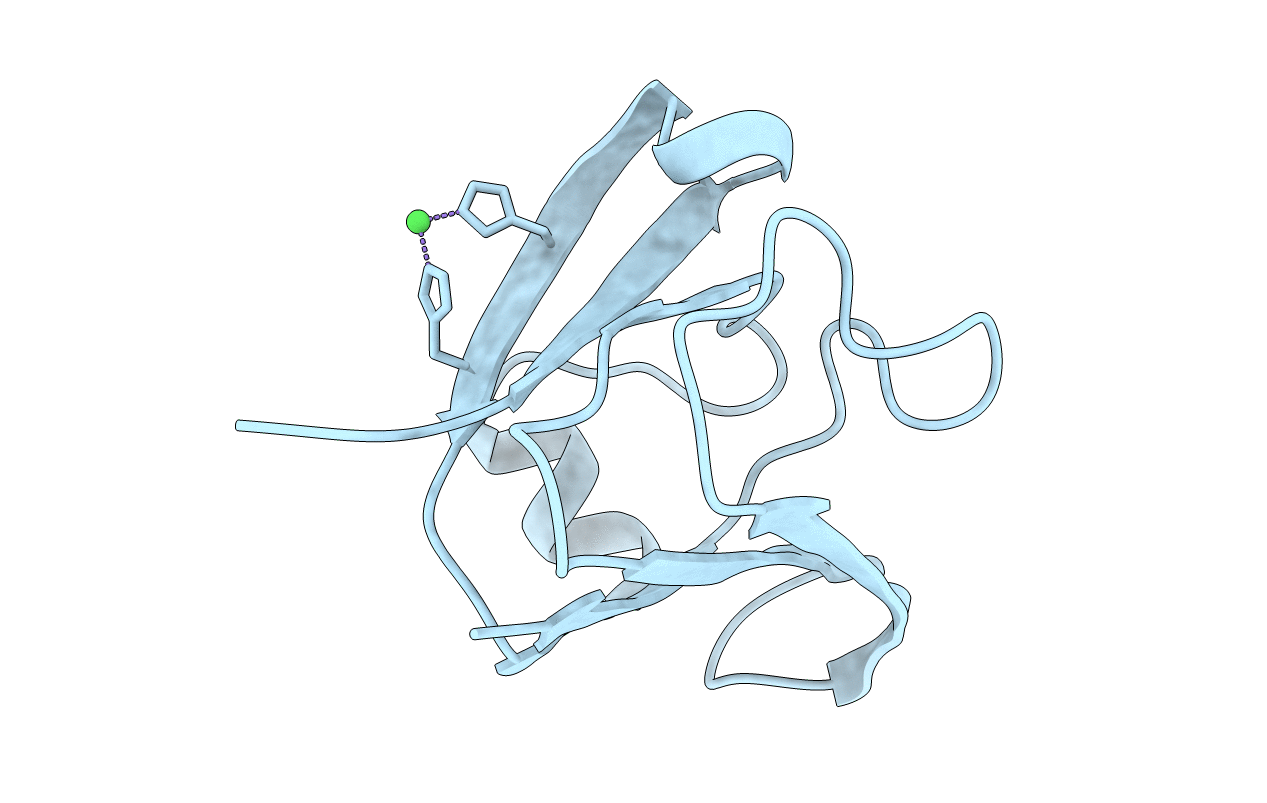
βγ-Crystallins are important constituents of the vertebrate eye lens, whereas in microbes, they are prevalent as Ca2+-binding proteins. In archaea, βγ-crystallins are conspicuously confined to two methanogens, viz., Methanosaeta and Methanosarcina. One of these, i.e., M-crystallin from Methanosarcina acetivorans, has been shown to be a typical Ca2+-binding βγ-crystallin. Here, with the aid of a high-resolution crystal structure and isothermal titration calorimetry, we report that "Methallin", a βγ-crystallin from Methanosaeta thermophila, is a trimeric, transition metal-binding protein. It binds Fe, Ni, Co, or Zn ion with nanomolar affinity, which is consistent even at 55 °C, the optimal temperature for the methanogen's growth. At the center of the protein trimer, the metal ion is coordinated by six histidines, two from each protomer, leading to an octahedral geometry. Small-angle X-ray scattering analysis confirms that the trimer seen in the crystal lattice is a biological assembly; this assembly dissociates to monomers upon removal of the metal ion. The introduction of two histidines (S17H/S19H) into a homologous βγ-crystallin, Clostrillin, allows it to bind nickel at the introduced site, though with micromolar affinity. However, because of the lack of a compatible interface, nickel binding could not induce trimerization, affirming that Methallin is a naturally occurring trimer for high-affinity transition metal binding. While βγ-crystallins are known to bind Ca2+ and form homodimers and oligomers, the transition metal-binding, trimeric Methallin is a new paradigm for βγ-crystallins. The distinct features of Methallin, such as nickel or iron binding, are also possible imprints of biogeochemical changes during the period of its origin.