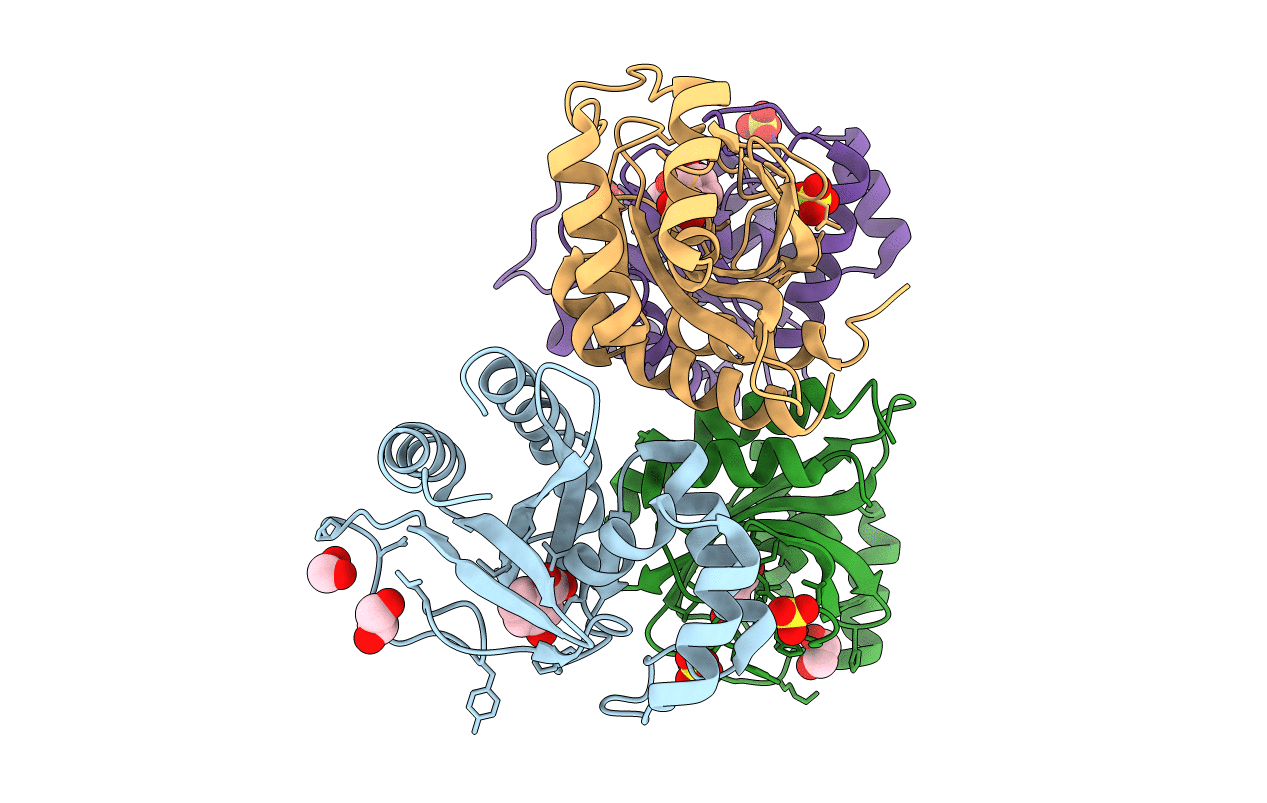
Deposition Date
2016-01-20
Release Date
2016-09-07
Last Version Date
2024-12-25
Entry Detail
PDB ID:
5HPI
Keywords:
Title:
Crystal Structure of the Double Mutant of PobR Transcription Factor Inducer Binding Domain-3-Hydroxy Benzoic Acid complex from Acinetobacter
Biological Source:
Source Organism(s):
Acinetobacter baylyi str. ADP1 (Taxon ID: 62977)
Expression System(s):
Method Details:
Experimental Method:
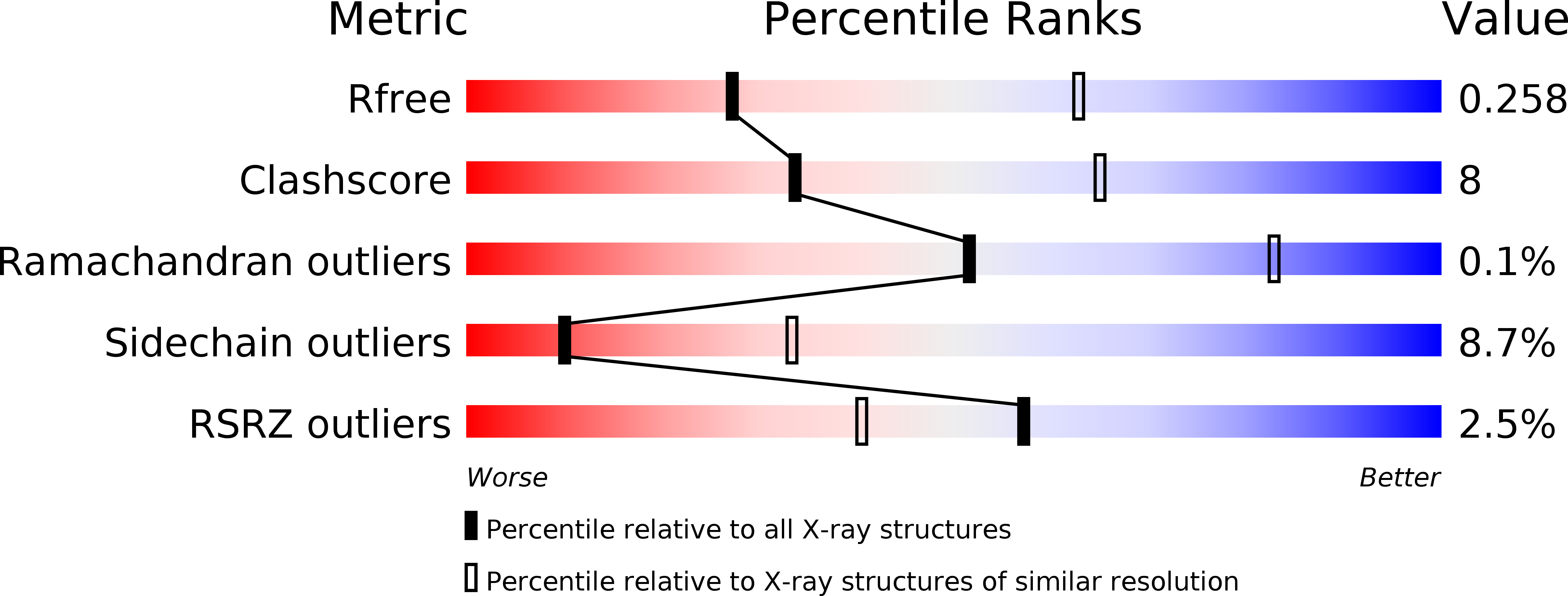
Resolution:
2.96 Å
R-Value Free:
0.25
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 2 2 21