Deposition Date
2016-01-07
Release Date
2016-02-24
Last Version Date
2023-11-15
Entry Detail
PDB ID:
5HG2
Keywords:
Title:
Backbone Modifications in the Protein GB1 Helix: beta-3-Ala24, beta-3-Lys28, beta-3-Lys31, beta-2-Asn35
Biological Source:
Source Organism(s):
Streptococcus sp. group G (Taxon ID: 1320)
Method Details:
Experimental Method:
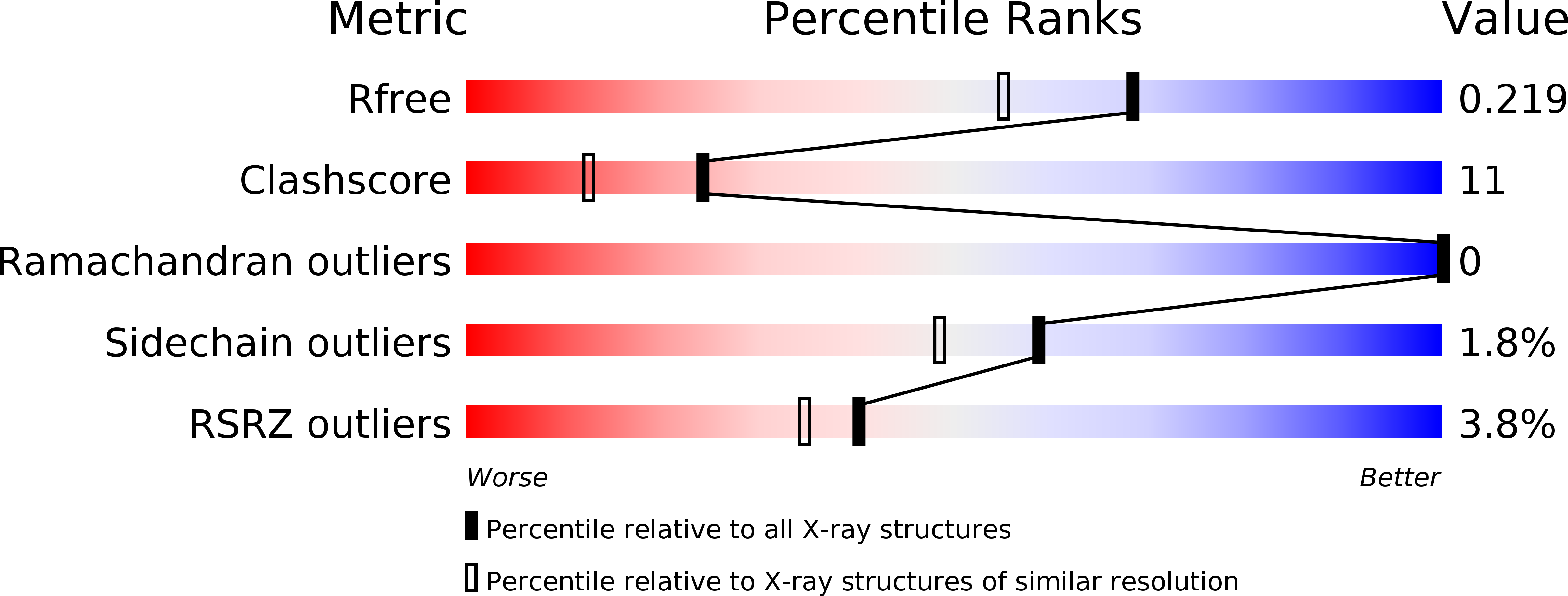
Resolution:
1.80 Å
R-Value Free:
0.21
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
P 41