Deposition Date
2016-01-02
Release Date
2016-04-13
Last Version Date
2023-09-27
Entry Detail
PDB ID:
5HBU
Keywords:
Title:
Structure of the E. coli nucleoid occlusion protein SlmA bound to DNA and the C-terminal tail of the cytoskeletal cell division protein FtsZ
Biological Source:
Source Organism(s):
Escherichia coli (strain K12) (Taxon ID: 83333)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
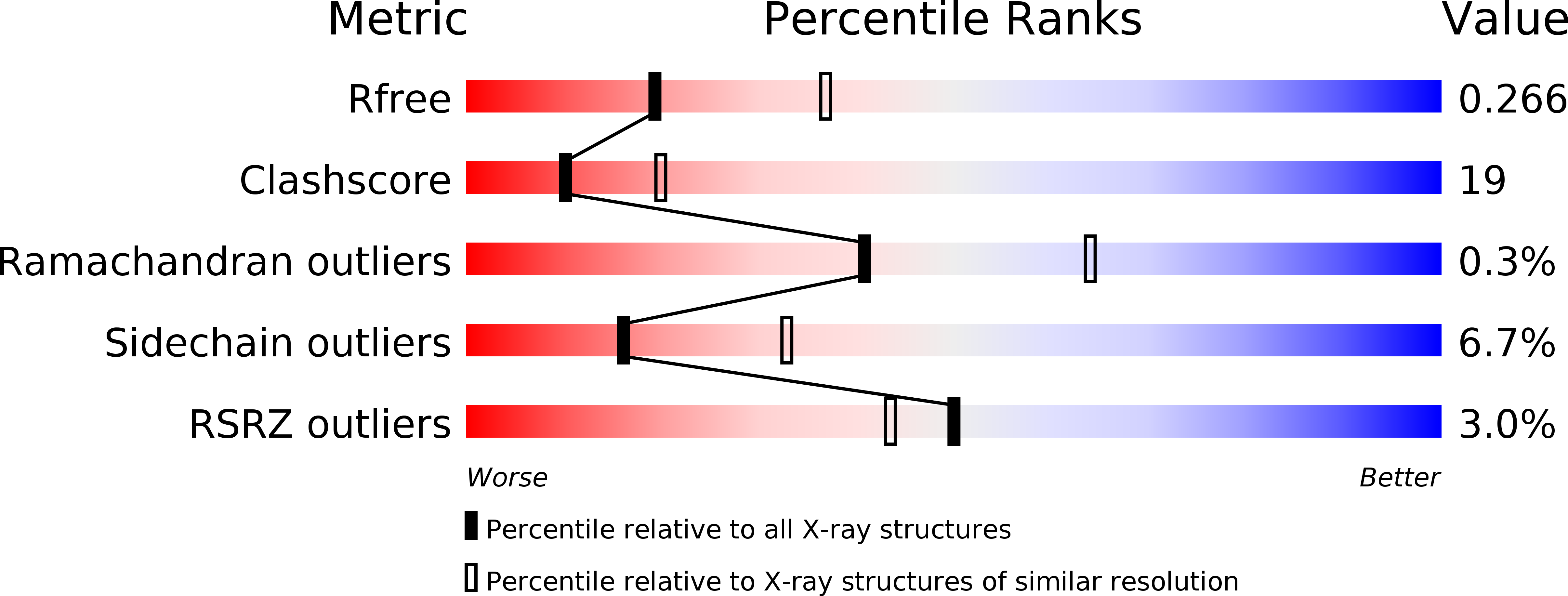
Resolution:
2.60 Å
R-Value Free:
0.26
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 21 21 21