Deposition Date
2015-12-31
Release Date
2016-04-13
Last Version Date
2024-05-22
Entry Detail
PDB ID:
5HAW
Keywords:
Title:
structures of the NO factor SlmA bound to DNA and the cytoskeletal cell division protein FtsZ
Biological Source:
Source Organism(s):
Vibrio cholerae serotype O1 (strain ATCC 39315 / El Tor Inaba N16961) (Taxon ID: 243277)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
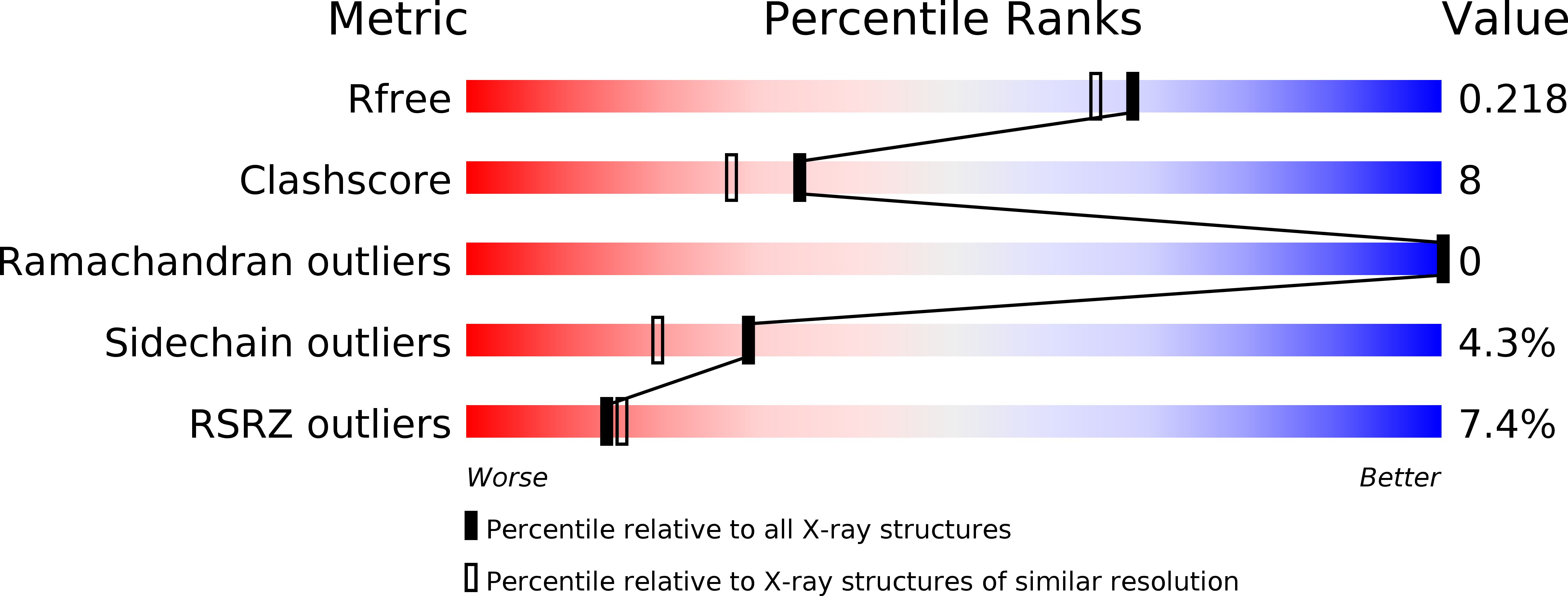
Resolution:
1.89 Å
R-Value Free:
0.21
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 31 2 1