Deposition Date
2016-05-23
Release Date
2017-04-05
Last Version Date
2024-01-10
Entry Detail
PDB ID:
5G5D
Keywords:
Title:
Crystal Structure of the CohScaC2-XDocCipA type II complex from Clostridium thermocellum
Biological Source:
Source Organism(s):
RUMINICLOSTRIDIUM THERMOCELLUM AD2 (Taxon ID: 1138384)
CLOSTRIDIUM THERMOCELLUM (Taxon ID: 203119)
CLOSTRIDIUM THERMOCELLUM (Taxon ID: 203119)
Expression System(s):
Method Details:
Experimental Method:
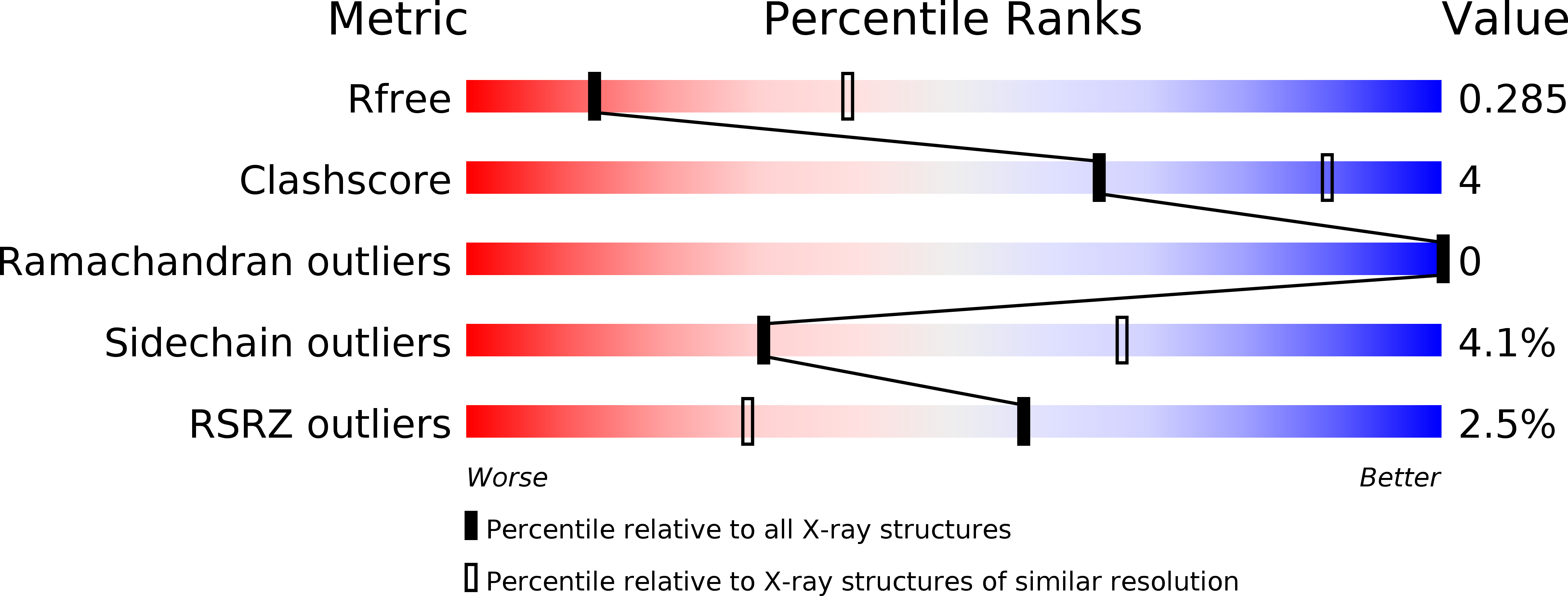
Resolution:
3.00 Å
R-Value Free:
0.30
R-Value Work:
0.25
R-Value Observed:
0.26
Space Group:
I 2 2 2